
Concept explainers
(a)
Interpretation: The Lewis structure for the given molecule should be determined. The electron-pair geometry and molecular geometry around central atom should be identified.
Concept Introduction:
- Lewis structures are diagrams that represent the
chemical bonding of covalently bonded molecules and coordination compounds. - It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Electron-Pair geometry: It is the geometry obtained by considering all valence electrons and bond pairs around central atom.
Molecular geometry: It is the geometry obtained by considering only the directly bonded atoms with the central atom.
(a)
Answer to Problem 18PS
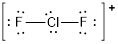
The electron pair geometry around chlorine is Trigonal bipyramidal and the molecular geometry is linear.
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.

The electron pair geometry around chlorine is Trigonal bipyramidal and the molecular geometry is linear since there are two bond pairs and three lone pairs around the chlorine atom.
(b)
Interpretation: The Lewis structure for the given molecule should be determined. The electron-pair geometry and molecular geometry around central atom should be identified.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Electron-Pair geometry: It is the geometry obtained by considering all valence electrons and bond pairs around central atom.
Molecular geometry: It is the geometry obtained by considering only the directly bonded atoms with the central atom.
(b)
Answer to Problem 18PS
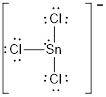
Electron pair geometry around
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.

Electron pair geometry around
(c)
Interpretation: The Lewis structure for the given molecule should be determined. The electron-pair geometry and molecular geometry around central atom should be identified.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Electron-Pair geometry: It is the geometry obtained by considering all valence electrons and bond pairs around central atom.
Molecular geometry: It is the geometry obtained by considering only the directly bonded atoms with the central atom.
(c)
Answer to Problem 18PS
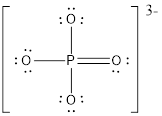
The electron pair and the molecular geometry around the central atom is tetrahedral.
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.

The electron pair and the molecular geometry around the central atom is tetrahedral since there are only four bond pairs and no lone pairs around the Phosphorus atom.
(d)
Interpretation: The Lewis structure for the given molecule should be determined. The electron-pair geometry and molecular geometry around central atom should be identified.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Electron-Pair geometry: It is the geometry obtained by considering all valence electrons and bond pairs around central atom.
Molecular geometry: It is the geometry obtained by considering only the directly bonded atoms with the central atom.
(d)
Answer to Problem 18PS

Electron pair geometry around C is linear. The molecular geometry is also linear.
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
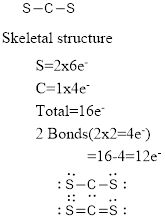
The electron pair geometry and the molecular geometry around carbon is linear since there are no lone pairs around the central atom.
Want to see more full solutions like this?
Chapter 8 Solutions
Owlv2 With Ebook, 1 Term (6 Months) Printed Access Card For Kotz/treichel/townsend/treichel's Chemistry & Chemical Reactivity, 10th
- > You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forwardConsider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forward
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
