
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 63E
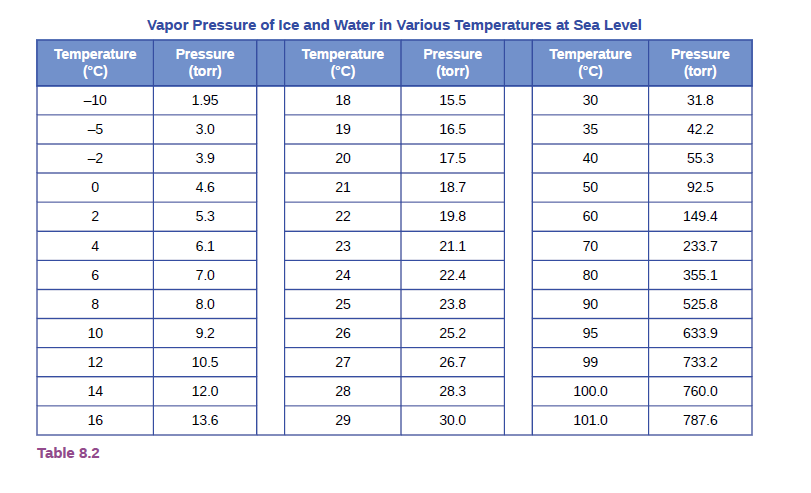
A sample of carbon monoxide was collected over water at a total pressure of 756 ton and a temperature of 18 °C. What is the pressure of the carbon monoxide? (See Table 8.2 for the vapor pressure of water.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 8 Solutions
Chemistry Atoms First2e
Ch. 8 - Why are sharp knives more effective than dull...Ch. 8 - Why do some small bridges have weight limits that...Ch. 8 - Why should you roll or belly-crawl rather than...Ch. 8 - A typical barometric pressure in Redding....Ch. 8 - A typical barometric pressure in Denver, Colorado,...Ch. 8 - A typical barometric pressure in Kansas City is...Ch. 8 - Canadian tire pressure gauges are marked in units...Ch. 8 - Dining the Viking landings on Mars, the...Ch. 8 - The pressure of the atmosphere on the surface of...Ch. 8 - A medical laboratory catalog describes the...
Ch. 8 - Consider this scenario and answer the following...Ch. 8 - Why is it necessary to use a nonvolatile liquid in...Ch. 8 - The pressure of a sample of gas is measured at sea...Ch. 8 - The pressure of a sample of gas is measured with...Ch. 8 - The pressure of a sample of gas is measured at sea...Ch. 8 - The pressure of a sample of gas ¡s measured a sea...Ch. 8 - How would the use of a volatile liquid affect the...Ch. 8 - Sometimes leaving a bicycle in the sun on a hot...Ch. 8 - Explain how the volume of the bubbles exhausted by...Ch. 8 - One way to state Boyle’s law is All other things...Ch. 8 - An alternate way to state Avogadro’s law is A1l...Ch. 8 - How would the graph in Figure 8.12 change if the...Ch. 8 - How would the graph in Figure 8.13 change if the...Ch. 8 - In addition to the data found in Figure 8.13, what...Ch. 8 - Determine the volume of 1 mol of CH4 gas at 150 K...Ch. 8 - Determine the pressure of the gas in the syringe...Ch. 8 - A spray can is used until it is empty except for...Ch. 8 - What is the temperature of an 11.2-L sample of...Ch. 8 - À 2.50-L volume of hydrogen measured at —196 C is...Ch. 8 - A balloon inflated with three breaths of air has a...Ch. 8 - A weather balloon contains 8.80 moles of helium at...Ch. 8 - The volume of an automobile air bag was 66.8 L...Ch. 8 - How many moles of gaseous boron trifluoride, BF3,...Ch. 8 - Iodine, I2, is a solid at room temperature but...Ch. 8 - How many grams of gas are present in each of the...Ch. 8 - A high altitude balloon is filled with 1041104 L...Ch. 8 - A cylinder of medical oxygen has a volume of 3S.4...Ch. 8 - A large scuba tank (Figure 8.16) with a volume of...Ch. 8 - A 20.0-L cylinder containing 11.34 kg of butane,...Ch. 8 - While resting, the average 70-kg human male...Ch. 8 - For a given amount of gas showing ideal behavior,...Ch. 8 - A liter of methane gas, CH4, at STP contains more...Ch. 8 - The effect of chlorofluorocarbons (such as CCl2F2)...Ch. 8 - As 1 g of (lie radioactive element radium decays...Ch. 8 - A balloon with a volume of 100.21 L at 21 C and...Ch. 8 - If the temperature of a fixed amount of a gas is...Ch. 8 - If the volume of a fixed amount of a gas is...Ch. 8 - What is the density of laughing gas, dinitrogen...Ch. 8 - Calculate the density of Freon 12, CF2Cl2, at 30.0...Ch. 8 - Which is denser at the same temperature and...Ch. 8 - A cylinder of O2(g) used in breathing by patients...Ch. 8 - What is the molar mass of a gas if 0.0494 g of the...Ch. 8 - What is the molar mass of a gas if 0.281 g of the...Ch. 8 - How could you show experimentally that the...Ch. 8 - The density of a certain gaseous fluoride of...Ch. 8 - Consider this question: What is the molecular...Ch. 8 - A 36.0—L cylinder of a gas used for calibration of...Ch. 8 - A cylinder of a gas mixture used for calibration...Ch. 8 - A sample of gas isolated from unrefined petroleum...Ch. 8 - A mixture of 0.200 g of 1.00 g of and 0.820 g of...Ch. 8 - Most mixtures of hydrogen gas with oxygen gas are...Ch. 8 - A commercial mercury vapor analyzer can detect in...Ch. 8 - A sample of carbon monoxide was collected over...Ch. 8 - In an experiment in a general chemistry...Ch. 8 - Joseph Priestley first prepared pure oxygen by...Ch. 8 - Cavendish prepared hydrogen in 176G by the novel...Ch. 8 - The chlorofluorocarbon CCl2F2 can be recycled into...Ch. 8 - Automobile air bags are inflated with nitrogen...Ch. 8 - Lime, CaO, is produced by heating calcium...Ch. 8 - Before small batteries were available, carbide...Ch. 8 - Calculate the volume of oxygen required to burn...Ch. 8 - What volume of O2 at STP is required to oxidize...Ch. 8 - Consider the following questions: (a) What is the...Ch. 8 - Methanol, CH3OH, is produced industrially by the...Ch. 8 - What volume of oxygen a 423.0 K and a pressure of...Ch. 8 - A 230-L sample of a colorless gas at STP...Ch. 8 - Ethanol, C2H5OH, is produced industrially from...Ch. 8 - One molecule of hemoglobin will combine with four...Ch. 8 - A sample of a compound of xenon and fluorine was...Ch. 8 - One method of analyzing amino acids is the van...Ch. 8 - A balloon filled with helium gas takes 6 hours to...Ch. 8 - Explain why the numbers of molecules are not...Ch. 8 - Starting with the definition of rate of effusion...Ch. 8 - Heavy water, D2O (molar mass = 20.03 g mol-1). can...Ch. 8 - Which of the following gases diffuse more slowly...Ch. 8 - During the discussion of gaseous diffusion for...Ch. 8 - Calculate the relative rate of diffusion of...Ch. 8 - A gas of unknown identity diffuses at a rate of...Ch. 8 - When two cotton plugs. one moistened with ammonia...Ch. 8 - Using the postulates of the kinetic molecular...Ch. 8 - Can the speed of a given molecule in a gas double...Ch. 8 - Describe what happens o the average kinetic energy...Ch. 8 - The distribution of molecular velocities in a...Ch. 8 - What is the ratio of the average kinetic energy of...Ch. 8 - A 1-L sample of CO initially at STP is heated to...Ch. 8 - The root mean square speed of H2, molecules at 25...Ch. 8 - Answer the following questions: (a) Is the...Ch. 8 - Show that the ratio of the rate of diffusion of...Ch. 8 - Graphs showing the behavior of several different...Ch. 8 - Explain why the plot of PV for CO2 differs from...Ch. 8 - Under which of the following sets of conditions...Ch. 8 - Describe the factors responsible for the deviation...Ch. 8 - For which of the following gases should the...Ch. 8 - A 0.245-L flask contains 0.467 mol CO2 at 159 C....Ch. 8 - Answer the following questions: (a) If XX behaved...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning