
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 55P
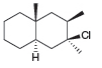
Draw all of the substitution and elimination products from the given
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Try: Draw possible resonance contributing structures for the following organic species:
CH3CH2NO2
[CH2CHCH2] [CH2CHCHO]
[CH2CHCH2]
[CH2CHNH2]
Complete the following synthesis.
(d). H+
ง
с
Can the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material?
If yes, draw the synthesis. Include all steps and all reactants.
Chapter 8 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
How does trandlation differ from transcription?
Microbiology: Principles and Explorations
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forward
- IX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forward
- Predict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardQ6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License