
(a)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of
For
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(a)
Answer to Problem 50PP
The systematic name for the molecule (a) is trans-3,4,5,5-tetramethyl-3-heptene.
Explanation of Solution
To identify: The systematic name for the given structure (a).
Draw the given molecule (a) and find the longest parent carbon chain or carbon skeleton and the substituents for naming the compound.
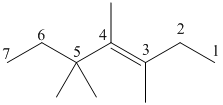
trans-3,4,5,5-tetramethyl-3-heptene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is the chain which contains 7 carbons. Hence the root name of the molecule is hept. Since it is an alkene with 7 carbons then heptene will be the parent carbon chain name.
The substituents present in the molecule are methyl groups on 3, 4 and 5. Therefore on numbering the three methyl groups are present at 3, 4 and 5, 5 positions is 3, 4, 5, 5-tetramethyl.
The bulky groups attached on the opposite sides of double bonded carbon atoms, so the given alkene molecule is ‘trans’.
Hence the systematic name for the molecule (a) is trans-3, 4, 5, 5-tetramethyl-3-heptene.
(b)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc...
For alkenes, suffix will be ‘ene’.
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(b)
Answer to Problem 50PP
The systemic name for the molecule (b) is 1-ethylcyclohexene.
Explanation of Solution
To identify: The systematic name for the given structure (b).
Draw the given molecule (b) and find the longest parent carbon chain or carbon skeleton and the substituents.
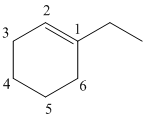
1-ethylcyclohexene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is cyclic in nature which consists of 6 carbons so the root word for the molecule is cyclohexane. The suffix ‘ene’ is used since it is an alkene.
The substituent present in the molecule are 1 ethyl group. Therefore it named as 1-ethyl in suffix.
Hence the systematic name for the molecule (b) is 1-ethylcyclohexene.
(c)
Interpretation:
For the given organic structures IUPAC name should be identified.
Concept Introduction
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc...
For alkenes, suffix will be ‘ene’.
Root word represents the longest continuous carbon skeleton of the organic molecule.
When a molecule consists of cyclic structure, the root word of the molecule is prefixed with cyclo, if it is two cyclic structure combined then prefixed with bicyclo.
Two stereoisomers are there for an alkene molecule. It depends upon the location of bulky group (or high molecular weight) on the double bonded carbon atoms. If the bulky groups are in same side then it is cis-isomer. If the bulky groups are in opposite side then it is trans-isomer.
(c)
Answer to Problem 50PP
The systematic name for molecule (c) is 2–methyl bicyclo [2 2 2] oct-2-ene.
Explanation of Solution
To identify: The systematic name for the given structure (c).
Draw the given molecule (d) and find the longest parent carbon chain or carbon skeleton and the substituents.
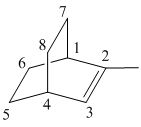
2–methylbicyclo [2 2 2] oct-2-ene
The given molecule is drawn. The parent carbon skeleton is the longest continuous carbon chain that should contain more number of carbons. In the given molecule the parent carbon skeleton is bicyclic in nature which consists of 8 carbons so the root word for the molecule is bicyclooctane. The suffix ‘ene’ is used since it is an alkene.
The given molecule is bicyclo fused alkene, number of carbons at the bridge and bridge head is counted and it is found that there are 2 carbons each at bridge head and 2 carbons at bridge.
The substituents present in the molecule are 1 methyl group. Therefore it named as 1-methyl in suffix.
Hence the systematic name for the molecule (c) is 2–methylbicyclo [2 2 2] oct-2-ene.
The systematic name for the given molecule is given by using the IUPAC rules.
Want to see more full solutions like this?
Chapter 8 Solutions
Student Study Guide and Solutions Manual T/A Organic Chemistry
- Complete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forward
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





