
Concept explainers
(a)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(a)
Explanation of Solution
The structure of
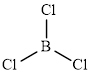
This molecule has a trigonal planar geometry. The direction dipole in the molecule is represented below,
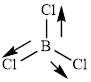
However
(b)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(b)
Explanation of Solution
The structure of
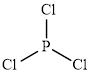
This molecule has a trigonal planar geometry. The direction dipole in the molecule is represented below,
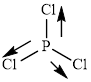
However
(c)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end (dipole) and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(c)
Explanation of Solution
The structure of
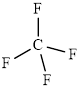
This molecule has a tetrahedral geometry. The direction dipole in the molecule is represented below,
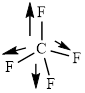
However
(d)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(d)
Explanation of Solution
The structure of
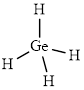
This molecule has a tetrahedral geometry. The direction dipole in the molecule is represented below,
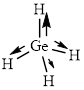
However
Want to see more full solutions like this?
Chapter 8 Solutions
CHEMISTRY+CHEM...(LL)-W/ACCESS >CUSTOM<
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning


