
(a)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a
Charged nucleophile is stronger than neutral nucleophiles.
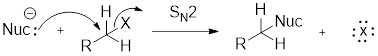
Structure of the substrate plays major role in the reactivity of
(b)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
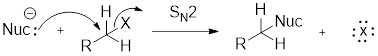
Structure of the substrate plays major role in the reactivity of
(c)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
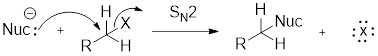
Structure of the substrate plays major role in the reactivity of
(d)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
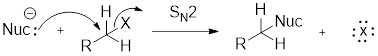
Structure of the substrate plays major role in the reactivity of
(e)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
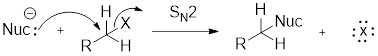
Structure of the substrate plays major role in the reactivity of
(f)
Interpretation:
Products formed when methyl bromide reacts with given nucleophile have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
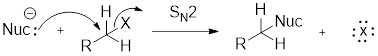
Structure of the substrate plays major role in the reactivity of
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- Identify as SN1 or SN2 and write the mechanism.arrow_forwardComplete the reaction. Not the mechanism.arrow_forwardDraw the mechanism using the arrows on conventions, including all formal charges and correct arrows. If stereochemical distinction can be made they should be included in the structure of the products.arrow_forward
- Draw the epoxide formed when the following alkene is treated with mCPBA. Click the "draw structure" button to launch the drawing utility. draw structure ...arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check CF3 (Choose one) OH (Choose one) H (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardIdentifying electron-donating and electron-withdrawing effects For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density CF3 O donating O donating O electron-rich O withdrawing withdrawing O no inductive effects O no resonance effects O electron-deficient O similar to benzene OCH3 Explanation Check O donating O donating ○ withdrawing withdrawing O no inductive effects no resonance effects electron-rich electron-deficient O similar to benzene Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- The acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardCUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





