
Concept explainers
Rank the
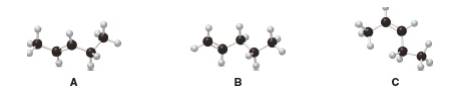
Interpretation: The given alkenes shown in the ball-and-sticks models are to be ranked in order of increasing stability.
Concept introduction: Stability of alkenes is governed by Zaitsev rule. More substituted alkenes are more stable.
Answer to Problem 24P
The stability order of given alkenes is
Explanation of Solution
According to Zaitsev rule the more substituted or more alkylated alkene is more stable which makes the order of stability as tetra-substituted > tri-substituted > di-substituted > mono-substituted.Trans alkenes are more stable than cis alkenes as there substitutions are attached far from each other which makes them less sterically hindered. In the given question A is trans-disubstitued alkene, B is mono-substituted alkene while C is cis-disubstitued alkene.
Explain how you got molecular structure from the given ball and stick model.
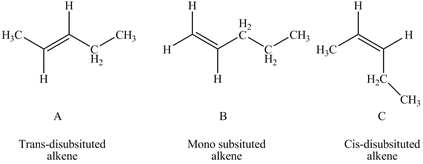
Figure 1
So, A and C are more stable than B.A is more stable than C as A is trans isomer while C is cis isomer. Therefore, stability order of given alkenes is
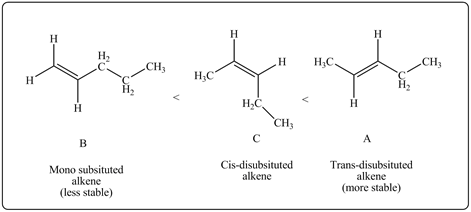
Figure 2
The stability order of given alkenes is–
Want to see more full solutions like this?
Chapter 8 Solutions
Connect Online Access 1-Semester for Organic Chemistry
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
General, Organic, and Biological Chemistry - 4th edition
Biology: Concepts and Investigations
HUMAN ANATOMY
Organic Chemistry (8th Edition)
- The molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forwardWhat characteristics should an interface that forms an electrode have?arrow_forward
- For a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forward
- State two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
