
Interpretation:
The purposeof the use of concentrated
Concept Introduction:
The preparation of aspirin takes place by the reaction of salicylic acid and acetic anhydride represented as follows:
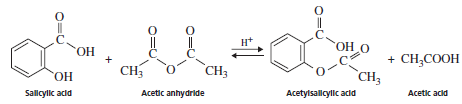
The -OH group of the salicylic acid reacts with acetic anhydride to form ester thus, the formation of aspirin is an esterification reaction.
Answer to Problem 1Q
To activate the reaction.
Explanation of Solution
The acid serves to protonate one double bonded oxygen on the acetic anhydride this makes the reaction with the salicylic acid faster.
The mechanism is represented as follows:
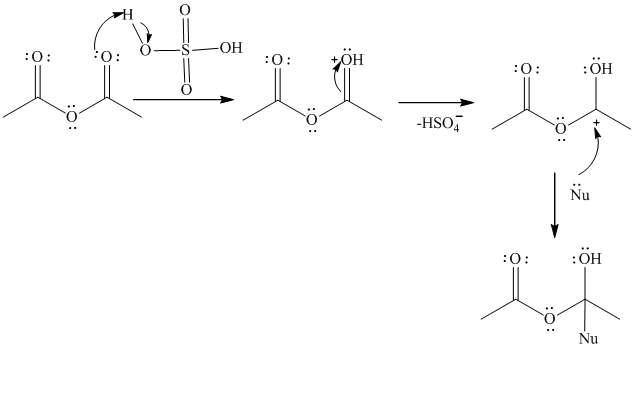
Thus, the acid acts as the catalyst during the esterification reaction by making the carbonyl group of anhydrous acetic acid more reactive.
Thus, the purpose of the use of concentrated
Want to see more full solutions like this?
Chapter 8 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Which of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forwardWhich of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forwardConsider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardConsider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forwardWhat is the missing reactant in this organic reaction? OH H + R Δ CH3-CH2-CH-CH3 O CH3 CH3-CH2-C-O-CH-CH2-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answe box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C O2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cerarrow_forward
- Predict the product of this organic reaction: CH3 NH2 Δ CH3-CH-CH3 + HO-C-CH2-N-CH3 P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. Xarrow_forwardIn the scope of the SCH4U course, please thoroughly go through the second questionarrow_forwardPlease help me solve these two problems. Thank you in advance.arrow_forward
- Naming and drawing unsubstituted esters Write the systematic name of each organic molecule: Explanation structure Check name Х 2/5arrow_forwardPredict the product of this organic reaction: =0 CH3-O-CH2-C-OH + CH3-OH H P+H₂O A Specifically, in the drawing area below draw the condensed structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. ☐arrow_forwardNaming and drawing USUsted ester Draw the condensed structure of ethyl hexanoate. Click anywhere to draw the first atom of your structure. × A : ☐arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





