
Consider the following particulate-level representation of a chemical equation:
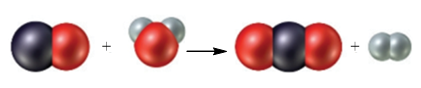
The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. (a) Write a balanced chemical equation representing this reaction. (b) Write a word description of the reaction on the particulate and molar levels.
(a)
Interpretation:
The balanced chemical equation representing the given particulate–level reaction is to be stated.
Concept introduction:
In a balanced chemical equation, all the reactants and products are written with their stoichiometric coefficients and their physical states. The number of atoms of an element on both sides of a balanced chemical equation is equal.
Answer to Problem 1E
The chemical equation that represents the given particulate–level reaction is shown below.
Explanation of Solution
The given reaction is,
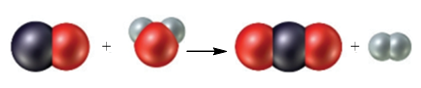
Figure 1
The black sphere represents carbon atom, white spheres represents hydrogen atom and red sphere represents oxygen atom. The chemical equation that represents the given particulate–level reaction is,
The given reaction is balanced as the number of atoms on both the sides of equation is same.
The chemical equation that represents the given particulate–level reaction is,
(b)
Interpretation:
The word description of the given particulate–level reaction is to be stated.
Concept introduction:
In a balanced chemical equation, all the reactants and products are written with their stoichiometric coefficients and their physical states. The number of atoms of an element on both sides of a balanced chemical equation is equal.
Answer to Problem 1E
The given particulate–level reaction involves the reaction of one molecule of carbon monoxide with one molecule of water resulting in the formation of one molecule of carbon dioxide and one molecule of hydrogen.
The given reaction at molar levels involves the reaction of one mole of carbon monoxide with one mole of water resulting in the formation of one mole of carbon dioxide and one mole of hydrogen.
Explanation of Solution
The chemical equation that represents the given particulate–level reaction is,
The given reaction is balanced as the number of atoms on both the sides of equation is same.
In the given reaction at particulate levels, one molecule of carbon monoxide reacts with one molecule of water to form one molecule of carbon dioxide and one molecule of hydrogen.
In the given reaction at molar levels, one mole of carbon monoxide reacts with one mole of water to form one mole of carbon dioxide and one mole of hydrogen.
The given particulate–level reaction involves the reaction of one molecule of carbon monoxide with one molecule of water resulting in the formation of one molecule of carbon dioxide and one molecule of hydrogen.
The given reaction at molar levels involves the reaction of one mole of carbon monoxide with one mole of water resulting in the formation of one mole of carbon dioxide and one mole of hydrogen.
Want to see more full solutions like this?
Chapter 8 Solutions
Introduction to Chemistry, Special Edition
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





