
EBK STUDY GUIDE TO ACCOMPANY CHEMISTRY:
7th Edition
ISBN: 9781119360889
Author: HYSLOP
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 134RQ
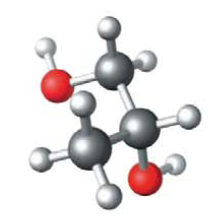
Below is a ball-and-stick model of a type of alcohol derived from a hydrocarbon. What is the formula for the hydrocarbon and what is its name?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part VII. The H-NMR of a compound with molecular formula C5 H 10 O2 is given below.
Find the following:
(a) The no. of protons corresponding to each signal in the spectra
(6) Give the structure of the compound and assign the signals to each
proton in the compound.
a
70.2
Integration Values
C5H10O2
b
47.7
C
46.5
d
69.5
3.6 3.5
3.4 3.3 3.2 3.1 3.0
2.9 2.8
2.7
2.6 2.5
2.4 2.3 2.2 2.1 2.0
Chemical Shift (ppm)
1.9
1.8
1.7 1.6
1.5
1.4 1.3 1.2
1.1 1.0
0.9 0.8
Part 111. 1 H-NMR spectrum of a compound with integration values in red is given below.
Answer the following:
(a) write the signals in the 'H-NMR spectrum to the corresponding protons on the structure
of the molecule below.
(b) Identify the theoretical multiplicities for each proton in the compound. Also give the possible.
complex splitting patterns assuming J values are not similar.
там
Br
22
2
3
6
4 7.2 7.0 6.8 6.6 6.4 6.2 6.0 5.8 5.6 5.4 5.2 5.0 4.8 4.6 4.4 4.2 4.0 3.8 3.6 3.4 3.2 3.0 2.8 2.6 2.4 2.2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0
Chemical Shift (ppm)
ra.
Br
2
3
6
6
2.5
2.4
2.3
2.2
2.1
2.0
1.9
1.8
1.7
1.6
1.5
1.4
1.3
1.2
1.1
1.0
0.9
0.8
Chemical Shift (ppm)
2
2
Br
7.3
7.2
7.1
7.0 6.9
6.7 6.6 6.5
6.4
6.3
6.2
6.1
6.0
Chemical Shift (ppm)
5.9
5.8 5.7
5.5 5.4 5.3 5.2
5.0 4.9
1600°
1538°C
1493°C
In the diagram, the letter L indicates
that it is a liquid. Indicate its
components in the upper region
where only L is indicated.
The
iron-iron carbide phase
diagram.
Temperature (°C)
1400
8
1394°C
y+L
1200
2.14
y, Austenite
10000
912°C
800a
0.76
0.022
600
400
(Fe)
a, Ferrite
Composition (at% C)
15
1147°C
a + Fe3C
2
3
Composition (wt% C)
L
2500
4.30
2000
y + Fe3C
727°C
1500
Cementite (Fe3C)
1000
4
5
6
6.70
Temperature (°F)
Chapter 8 Solutions
EBK STUDY GUIDE TO ACCOMPANY CHEMISTRY:
Ch. 8 - Practice Exercise 8.1
Choose the ionic compound...Ch. 8 - Construct an energy diagram similar to the one in...Ch. 8 - What is wrong with the following electron...Ch. 8 - How do the electron configurations change when a...Ch. 8 - Practice Exercise 8.5
How are the electron...Ch. 8 - Practice Exercise 8.6
Draw the Lewis structures...Ch. 8 - Practice Exercise 8.7
Use Lewis symbols to diagram...Ch. 8 - Prob. 8PECh. 8 - Prob. 9PECh. 8 - For each atom that does not have an octet, how...
Ch. 8 - The chlorine end of the chlorine monoxide molecule...Ch. 8 - Although isolated Na+ and Cl- ions are unstable,...Ch. 8 - Bromine and chlorine form a molecular substance...Ch. 8 - Practice Exercise 8.14
For each of the following...Ch. 8 - Prob. 15PECh. 8 - Draw Lewis structures for...Ch. 8 - Practice Exercise 8.17
Using the structures drawn...Ch. 8 - Practice Exercise 8.18
A student drew the...Ch. 8 - Assign formal charges to the atoms in the...Ch. 8 - Practice Exercise 8.20
Draw the preferred Lewis...Ch. 8 - Prob. 21PECh. 8 - Practice Exercise 8.22
Use Lewis structures to...Ch. 8 - Prob. 23PECh. 8 - Practice Exercise 8.24
Draw the resonance...Ch. 8 - Determine the preferred Lewis structure for the...Ch. 8 - Prob. 26PECh. 8 - Practice Exercise 8.27
The following questions...Ch. 8 - What must be true about the change in the total...Ch. 8 - 8.2 Under what conditions could a compound form...Ch. 8 - 8.3 What is an ionic bond?
Ch. 8 - Define the term lattice energy. In what ways does...Ch. 8 - How is the tendency to form ionic bonds related to...Ch. 8 - What influence do ion size and charge have on...Ch. 8 - 8.7 What is the octet rule? What is responsible...Ch. 8 - 8.8 Why doesn’t hydrogen obey the octet rule?
Ch. 8 - 8.9 Magnesium forms compounds containing the ion ...Ch. 8 - Prob. 10RQCh. 8 - Why do many of the transition elements in Period 4...Ch. 8 - Prob. 12RQCh. 8 - Prob. 13RQCh. 8 - Which of these Lewis symbols is incorrect?Ch. 8 - Define bond length and bond energy.Ch. 8 - 8.16 Define bond order. How are bond energy and...Ch. 8 - 8.17 The energy required to break the H—Cl bond to...Ch. 8 - In terms of the potential energy change, why...Ch. 8 - Prob. 19RQCh. 8 - Describe what happens to the electron density...Ch. 8 - Is the formation of a covalent bond endothermic or...Ch. 8 - What factors control the bond length in a covalent...Ch. 8 - How many covalent bonds are normally formed by (a)...Ch. 8 - What is a polar covalent bond?Ch. 8 - Define dipole moment in the form of an equation....Ch. 8 - 8.26 Define electronegativity. On what basis did...Ch. 8 - Which element has the highest electronegativity?...Ch. 8 - Prob. 28RQCh. 8 - If an element has a low electronegativity, is it...Ch. 8 - In what groups in the periodic table are the most...Ch. 8 - How is the electronegativity of a metal related to...Ch. 8 - 8.32 When we say that aluminum is more reactive...Ch. 8 - Arrange the following metals in their approximate...Ch. 8 - 8.34 Complete and balance the following equations....Ch. 8 - Prob. 35RQCh. 8 - Without looking at the text, describe the steps...Ch. 8 - 8.37 Why do we usually place the least...Ch. 8 - Why do Period 2 elements never form more than four...Ch. 8 - Define (a) single bond, (b) double bond, and (c)...Ch. 8 - Prob. 40RQCh. 8 - How many electrons are in the valence shells of...Ch. 8 - What is the minimum number of electrons that would...Ch. 8 - 8.43 Nitrogen and arsenic are in the same group in...Ch. 8 - 8.44 What is the definition of formal charge? How...Ch. 8 - How are formal charges for atoms in a molecule...Ch. 8 - 8.46 How are formal charges used to select the...Ch. 8 - 8.47 What are the formal charges on the atoms in...Ch. 8 - What is a coordinate covalent bond?Ch. 8 - Once formed, how (if at all) does a coordinate...Ch. 8 - BC13 has an incomplete valence shell. Use Lewis...Ch. 8 - Prob. 51RQCh. 8 - 8.52 What is a resonance hybrid? How does it...Ch. 8 - Prob. 53RQCh. 8 - Polystyrene plastic is a hydrocarbon that consists...Ch. 8 - Sketch the structures for (a) methane, (b) ethane,...Ch. 8 - Draw the structure for a hydrocarbon that has a...Ch. 8 - How many different molecules have the formula...Ch. 8 - What is a carbonyl group? In which classes of...Ch. 8 - Prob. 59RQCh. 8 - 8.60 Write a chemical equation for the ionization...Ch. 8 - Match the compounds on the left with the family...Ch. 8 - Prob. 62RQCh. 8 - In each of the following pairs of compounds, which...Ch. 8 - In each of the following pairs of compounds, which...Ch. 8 - Prob. 65RQCh. 8 - *8.66 Use an enthalpy diagram to calculate the...Ch. 8 - Explain what happens to the electron...Ch. 8 - Describe what happens to the electron...Ch. 8 - 8.69 What are the electron configurations of the ...Ch. 8 - 8.70 What are the electron configurations of the ...Ch. 8 - Write the abbreviated electron configuration of...Ch. 8 - Write the abbreviated electron configuration of...Ch. 8 - Prob. 73RQCh. 8 - Prob. 74RQCh. 8 - Prob. 75RQCh. 8 - Prob. 76RQCh. 8 - How much energy, in joules, is required to break...Ch. 8 - How much energy is released in the formation of...Ch. 8 - The reason there is danger in exposure to...Ch. 8 - A mixture of H2andCl2 is stable, but a bright...Ch. 8 - Prob. 81RQCh. 8 - Use Lewis structures to diagram the formation of...Ch. 8 - Chlorine tends to form only one covalent bond...Ch. 8 - Use the octet rule to predict the formula of the...Ch. 8 - Prob. 85RQCh. 8 - What would be the formula for the simplest...Ch. 8 - 8.87 Use the data in Table 8.3 to calculate the...Ch. 8 - The molecule bromine monofluoride has a dipole...Ch. 8 - Prob. 89RQCh. 8 - 8.90 The dipole moment of HF is 1.83 D and the...Ch. 8 - Prob. 91RQCh. 8 - Prob. 92RQCh. 8 - Which of the bonds in Problem 8.91 is the most...Ch. 8 - 8.94 Which of the bonds in the Problem 8.92 is the...Ch. 8 - Draw Lewis structures for...Ch. 8 - Draw Lewis structures for...Ch. 8 - Prob. 97RQCh. 8 - 8.98 Draw Lewis structures for
Ch. 8 - 8.99 Draw Lewis structures for (a) carbon...Ch. 8 - Draw Lewis structures for (a) selenium trioxide,...Ch. 8 - Prob. 101RQCh. 8 - 8.102 Draw Lewis structures for .
Ch. 8 - Draw the Lewis structure for (a) CH2O (the central...Ch. 8 - Draw Lewis structures for (a) the peroxide ion,...Ch. 8 - Assign formal charges to each atom in the...Ch. 8 - 8.106 Assign formal charges to each atom in the...Ch. 8 - Draw the Lewis structure for HCIO4. Assign formal...Ch. 8 - Draw the Lewis structure for SOCl2 (sulfur bonded...Ch. 8 - Prob. 109RQCh. 8 - 8.110 The following are two Lewis structures that...Ch. 8 - 8.111 Use Lewis structures to show that the...Ch. 8 - Use Lewis structures to show that the reaction...Ch. 8 - Draw all of the resonance structures for the N2O4...Ch. 8 - Prob. 114RQCh. 8 - How should the NO bond lengths compare in the NO3...Ch. 8 - Arrange the following in order of increasing CO...Ch. 8 - 8.117 The Lewis structure of was given as
but...Ch. 8 - *8.118 Use formal charges to establish the...Ch. 8 - 8.119 Give the formula and name of four different...Ch. 8 - Use data from the tables of ionization energies...Ch. 8 - 8.121 Changing to gaseous atoms requires a total...Ch. 8 - In many ways, tin(IV) chloride behaves more like a...Ch. 8 - In each pair, choose the one with the more polar...Ch. 8 - How many electrons are in the outer shell of the...Ch. 8 - Prob. 125RQCh. 8 - 8.126 Are the following Lewis structures...Ch. 8 - Assign formal charges to all the atoms in the...Ch. 8 - 8.128 Assign formal charges to all the atoms in...Ch. 8 - The inflation of an air bag when a car experiences...Ch. 8 - 8.130 How should the sulfur-oxygen bond lengths...Ch. 8 - What is the most reasonable Lewis structure for...Ch. 8 - Prob. 132RQCh. 8 - Prob. 133RQCh. 8 - 8.134 Below is a ball-and-stick model of a type of...Ch. 8 - 8.135 Explain why ions of the representative...Ch. 8 - Use Lewis structures to show the ionization of the...Ch. 8 - The compound below, an amine, is a weak base and...Ch. 8 - 8.138 Use Lewis structures to diagram the reaction...Ch. 8 - How many grams of water could have its temperature...Ch. 8 - Prob. 140RQCh. 8 - A 38.40 mg sample of an organic acid composed of...Ch. 8 - What is the average bond energy of a CC covalent...Ch. 8 - One way of estimating the electronegativity of an...Ch. 8 - 8.144 The attractions between molecules of a...Ch. 8 - The positive end of the dipole in a water molecule...Ch. 8 - In describing the structures of molecules, we use...
Additional Science Textbook Solutions
Find more solutions based on key concepts
29. A gas is compressed by an isothermal process that decreases its volume by a factor of 2. In this process, t...
College Physics: A Strategic Approach (3rd Edition)
1. Write a single sentence, using no more than 25 words, to summarize each of the following cellular processes...
Human Anatomy & Physiology (2nd Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Describe how the structure of the following connective tissue is related to its function: areolar connective ti...
Principles of Anatomy and Physiology
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardPart II. Given below are the 'H-NMR spectrum at 300 MHz in CDC13 and mass spectrum using electron ionization of compound Brian. The FTIR of the said compound showed a strong peak at 1710 cm"). Determine the following: (a) molecular Formula and Degree of unsaturation of compound Brian (b) Basing on the given H-NMR spectrum tabulate the following (i) chemical shifts (ii) integration, ciii) multiplicity and (iv) interferences made for each signal (c) Draw the structure of compound Brian. ) ΕΙ 43 41 27 71 114 (M+) Hmmm 20 30 40 50 60 70 80 90 100 110 120 1H NMR spectrum 300 MHz in CDCl3 2.0 alle 1.0arrow_forwardThe iron-iron carbide phase diagram. In the diagram, the letter L indicates that it is a liquid. Indicate what its components are. Temperature (°C) 1600 10 Composition (at% C) 15 25 1538°C -1493°C 8 1400 1200 1394°C y+L L 2500 1147°C y. Austenite 2.14 4.30 2000 1000 912°C y + Fe3C 800ㅏ 0.76 0.022 600 a, Ferrite a + Fe3C 400 0 (Fe) Composition (wt% C) 727°C 1500 Cementite (Fe3C) 1000 6 6.70 Temperature (°F)arrow_forward
- Part V. Choose which isomer would give the 1H-NMR spectrum below. Justify your reasoning by assigning important signals to the Corresponding protons of the correct molecule. A D on of of of H H 88 2 90 7.8 7.6 7.4 80 5 6 [ppm] 7.2 6.8 6.6 6.4 ō [ppm]arrow_forwardShow work with explanation. don't give Ai generated solutionarrow_forwardQ7. a. Draw the line-bond structure of the major product for the following reaction, if a reaction occurs, assume monohalogenation. b. Calculate the product ratios using the following information (hint: use the number of hydrogens in each category present to calculate the ratios). Chlorination: 1° Reactivity=1 2° Reactivity=4 Heat + Cl2 3° Reactivity=5arrow_forward
- Please correct answer and don't use hand rating and don't use Ai solutionarrow_forwardQ10: Alkane halogenation a. Give the name and structures of the five isomeric hexanes. Page 4 of 5 Chem 0310 Organic Chemistry 1 Recitations b. For each isomer, give all the free radical monochlorination and monobromination products that are structurally isomeric.arrow_forwardQ9. The insecticide DDT (in the box below) is useful in controlling mosquito populations and has low toxicity to humans, but is dangerous to birds and fish. Hoping to alleviate the dangers, little Johnny Whizbang, an aspiring chemist, proposes a new version of DDT ("Bromo-DDT") and shows his synthesis to his boss. Will Johnny Whizbang's synthesis work? Or will he be fired? Assume there is an excess of bromine and polybrominated products can be separated. Explain why. CH3 Br2, light CBR3 ok-ok Br Br Br Br CI "Bromo-DDT" CCl 3 DDT (dichlorodiphenyltrichloroethane) CIarrow_forward
- Differentiate the terms Monotectic, Eutectic, Eutectoid, Peritectic, Peritectoid.arrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, lightarrow_forwarda. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (a) (c) H3C CH3 .CH3 CH3 CH3 (b) Page 1 of 5 Chem 0310 Organic Chemistry 1 Recitations b. Draw all the possible radical products for 2-methylbutane, and determine which bond is most likely to be broken.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY