
Concept explainers
(a)
Interpretation: The systematic name of the given organic compound is to be interpreted.
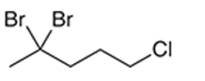
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of the chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a
(b)
Interpretation: The systematic name of the given organic compound is to be interpreted.
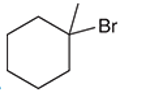
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(c)
Interpretation: The systematic name of the given organic compound is to be interpreted.
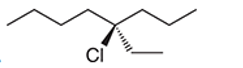
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(d)
Interpretation: The systematic name of the given organic compound is to be interpreted.
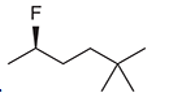
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(e)
Interpretation: The systematic name of the given organic compound is to be interpreted.
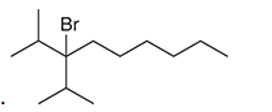
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(f)
Interpretation: The systematic name of the given organic compound is to be interpreted.
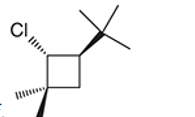
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The primary suffix in the root word indicates the presence of the single, double and triple bond in the molecule whereas the secondary suffix indicates the presence of a functional group in the molecule.
(g)
Interpretation: The systematic name of the given organic compound is to be interpreted.
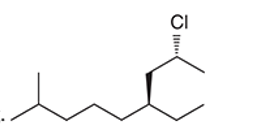
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
(h)
Interpretation: The systematic name of the given organic compound is to be interpreted.
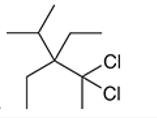
Concept Introduction: The IUPAC purposed the naming of organic and inorganic compounds. The name of chemical compounds can be given with the help of certain rules. To name an organic compound, the longest carbon chain must be selected as the parent chain and the substituent must be marked as the prefix. The single, double, and triple bonds in a molecule are indicated by the primary suffix in the root word, but a functional group is indicated by the secondary suffix.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ORGANIC CHEMISTRY (LL) W/ACCESS
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- What would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




