
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
8th Edition
ISBN: 9781307434316
Author: CENGEL
Publisher: INTER MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.13, Problem 144P
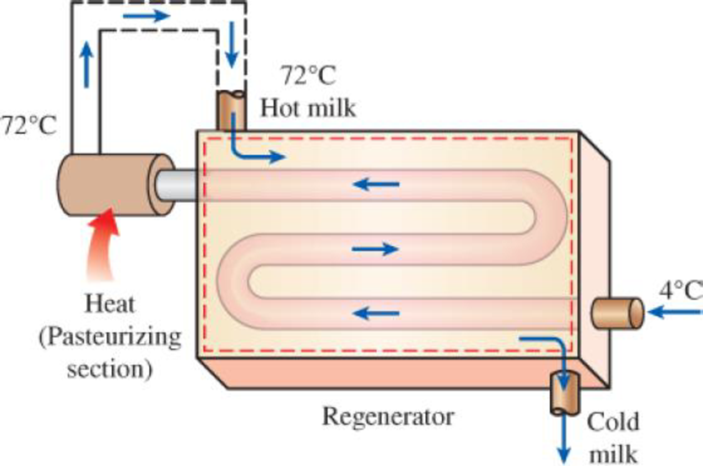
In a dairy plant, milk at 4°C is pasteurized continuously at 72°C at a rate of 12 L/s for 24 hours a day and 365 days a year. The milk is heated to the pasteurizing temperature by hot water heated in a natural-gas-fired boiler that has an efficiency of 82 percent. The pasteurized milk is then cooled by cold water at 18°C before it is finally refrigerated back to 4°C. To save energy and money, the plant installs a regenerator that has an effectiveness of 82 percent. If the cost of natural gas is $1.30/therm (1 therm = 105,500 kJ), determine how much energy and money the regenerator will save this company per year and the annual reduction in entropy generation.
FIGURE P7–143
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
HW8
A shaft fitted with a flywheel rotates at 650 r.p.m. and drives a machine. The
torque of machine varies in a cyclic manner over a period of 2 revolutions. The
torque rises from 650 N-m to 2200 N-m uniformly during 110° and remains
constant for the following 270°. It then falls uniformly to 600 N-m during the next
100° and remains constant for the end cycle, the cycle being repeated thereafter.
Determine the power required to drive the machine and percentage fluctuation in
speed, if the driving torque applied to the shaft is constant and the mass of the
flywheel is 180 kg with radius of gyration of 35 cm.
HW9
units of h. show all work
4. Steam flows steadily through a turbine at a rate of 47,000 lbm/h, entering at 1000 psia
and 800°F and leaving at 6 psia as saturated vapor. If the power generated by the
turbine is 3.7 MW, determine the rate of heat loss from the steam.
Chapter 7 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
Ch. 7.13 - Prob. 1PCh. 7.13 - Does the cyclic integral of heat have to be zero...Ch. 7.13 - Is a quantity whose cyclic integral is zero...Ch. 7.13 - Prob. 4PCh. 7.13 - Prob. 5PCh. 7.13 - How do the values of the integral 12Q/T compare...Ch. 7.13 - The entropy of a hot baked potato decreases as it...Ch. 7.13 - When a system is adiabatic, what can be said about...Ch. 7.13 - Prob. 9PCh. 7.13 - A pistoncylinder device contains helium gas....
Ch. 7.13 - A pistoncylinder device contains nitrogen gas....Ch. 7.13 - A pistoncylinder device contains superheated...Ch. 7.13 - The entropy of steam will (increase, decrease,...Ch. 7.13 - Prob. 14PCh. 7.13 - Prob. 15PCh. 7.13 - Prob. 16PCh. 7.13 - Steam is accelerated as it flows through an actual...Ch. 7.13 - Prob. 18PCh. 7.13 - Prob. 19PCh. 7.13 - Prob. 20PCh. 7.13 - Heat in the amount of 100 kJ is transferred...Ch. 7.13 - In Prob. 719, assume that the heat is transferred...Ch. 7.13 - 7–23 A completely reversible heat pump produces...Ch. 7.13 - During the isothermal heat addition process of a...Ch. 7.13 - Prob. 25PCh. 7.13 - During the isothermal heat rejection process of a...Ch. 7.13 - Prob. 27PCh. 7.13 - Prob. 28PCh. 7.13 - Two lbm of water at 300 psia fill a weighted...Ch. 7.13 - A well-insulated rigid tank contains 3 kg of a...Ch. 7.13 - The radiator of a steam heating system has a...Ch. 7.13 - A rigid tank is divided into two equal parts by a...Ch. 7.13 - 7–33 An insulated piston–cylinder device contains...Ch. 7.13 - Prob. 34PCh. 7.13 - Prob. 35PCh. 7.13 - Onekg of R-134a initially at 600 kPa and 25C...Ch. 7.13 - Refrigerant-134a is expanded isentropically from...Ch. 7.13 - Prob. 38PCh. 7.13 - Refrigerant-134a at 320 kPa and 40C undergoes an...Ch. 7.13 - A rigid tank contains 5 kg of saturated vapor...Ch. 7.13 - A 0.5-m3 rigid tank contains refrigerant-134a...Ch. 7.13 - Prob. 44PCh. 7.13 - Prob. 45PCh. 7.13 - Steam enters an adiabatic diffuser at 150 kPa and...Ch. 7.13 - Prob. 47PCh. 7.13 - An isentropic steam turbine processes 2 kg/s of...Ch. 7.13 - Prob. 50PCh. 7.13 -
7–51 0.7-kg of R-134a is expanded isentropically...Ch. 7.13 - Twokg of saturated water vapor at 600 kPa are...Ch. 7.13 - Steam enters a steady-flow adiabatic nozzle with a...Ch. 7.13 - Prob. 54PCh. 7.13 - In Prob. 755, the water is stirred at the same...Ch. 7.13 - A pistoncylinder device contains 5 kg of steam at...Ch. 7.13 - Prob. 57PCh. 7.13 - Prob. 59PCh. 7.13 - A 50-kg copper block initially at 140C is dropped...Ch. 7.13 - Prob. 61PCh. 7.13 - Prob. 62PCh. 7.13 - A 30-kg aluminum block initially at 140C is...Ch. 7.13 - A 30-kg iron block and a 40-kg copper block, both...Ch. 7.13 - An adiabatic pump is to be used to compress...Ch. 7.13 - Prob. 67PCh. 7.13 - Can the entropy of an ideal gas change during an...Ch. 7.13 - An ideal gas undergoes a process between two...Ch. 7.13 - Prob. 72PCh. 7.13 - Prob. 73PCh. 7.13 - Prob. 74PCh. 7.13 - Prob. 75PCh. 7.13 - A 1.5-m3 insulated rigid tank contains 2.7 kg of...Ch. 7.13 - An insulated pistoncylinder device initially...Ch. 7.13 - A pistoncylinder device contains 0.75 kg of...Ch. 7.13 - Prob. 80PCh. 7.13 - 7–81 Air enters a nozzle steadily at 280 kPa and...Ch. 7.13 - A mass of 25 lbm of helium undergoes a process...Ch. 7.13 - One kg of air at 200 kPa and 127C is contained in...Ch. 7.13 - Prob. 85PCh. 7.13 - Air at 3.5 MPa and 500C is expanded in an...Ch. 7.13 -
7–87E Air is compressed in an isentropic...Ch. 7.13 - An insulated rigid tank is divided into two equal...Ch. 7.13 - An insulated rigid tank contains 4 kg of argon gas...Ch. 7.13 - Prob. 90PCh. 7.13 - Prob. 91PCh. 7.13 - Prob. 92PCh. 7.13 - Air at 27C and 100 kPa is contained in a...Ch. 7.13 - Prob. 94PCh. 7.13 - Helium gas is compressed from 90 kPa and 30C to...Ch. 7.13 - Five kg of air at 427C and 600 kPa are contained...Ch. 7.13 - Prob. 97PCh. 7.13 - The well-insulated container shown in Fig. P 795E...Ch. 7.13 - Prob. 99PCh. 7.13 - Prob. 100PCh. 7.13 - It is well known that the power consumed by a...Ch. 7.13 - Prob. 102PCh. 7.13 - Prob. 103PCh. 7.13 - Saturated water vapor at 150C is compressed in a...Ch. 7.13 - Liquid water at 120 kPa enters a 7-kW pump where...Ch. 7.13 - Prob. 106PCh. 7.13 - Consider a steam power plant that operates between...Ch. 7.13 - Helium gas is compressed from 16 psia and 85F to...Ch. 7.13 - Nitrogen gas is compressed from 80 kPa and 27C to...Ch. 7.13 - Saturated refrigerant-134a vapor at 15 psia is...Ch. 7.13 - Describe the ideal process for an (a) adiabatic...Ch. 7.13 - Is the isentropic process a suitable model for...Ch. 7.13 - On a T-s diagram, does the actual exit state...Ch. 7.13 - Steam at 100 psia and 650F is expanded...Ch. 7.13 - Prob. 117PCh. 7.13 - Combustion gases enter an adiabatic gas turbine at...Ch. 7.13 - Steam at 4 MPa and 350C is expanded in an...Ch. 7.13 - Prob. 120PCh. 7.13 - Prob. 122PCh. 7.13 - Prob. 123PCh. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - Prob. 126PCh. 7.13 - Argon gas enters an adiabatic compressor at 14...Ch. 7.13 - Air enters an adiabatic nozzle at 45 psia and 940F...Ch. 7.13 - Prob. 130PCh. 7.13 - An adiabatic diffuser at the inlet of a jet engine...Ch. 7.13 - Hot combustion gases enter the nozzle of a...Ch. 7.13 - Refrigerant-134a is expanded adiabatically from...Ch. 7.13 - Oxygen enters an insulated 12-cm-diameter pipe...Ch. 7.13 - Prob. 135PCh. 7.13 - Prob. 136PCh. 7.13 - Steam enters an adiabatic turbine steadily at 7...Ch. 7.13 - 7–138 In an ice-making plant, water at 0°C is...Ch. 7.13 - Water at 20 psia and 50F enters a mixing chamber...Ch. 7.13 - Prob. 140PCh. 7.13 - Prob. 141PCh. 7.13 - Prob. 142PCh. 7.13 - Prob. 143PCh. 7.13 - In a dairy plant, milk at 4C is pasteurized...Ch. 7.13 - An ordinary egg can be approximated as a...Ch. 7.13 - Prob. 146PCh. 7.13 - Prob. 147PCh. 7.13 - In a production facility, 1.2-in-thick, 2-ft 2-ft...Ch. 7.13 - Prob. 149PCh. 7.13 - Prob. 150PCh. 7.13 - A frictionless pistoncylinder device contains...Ch. 7.13 - Prob. 152PCh. 7.13 - Prob. 153PCh. 7.13 - Prob. 154PCh. 7.13 - Prob. 155PCh. 7.13 - Liquid water at 200 kPa and 15C is heated in a...Ch. 7.13 - Prob. 157PCh. 7.13 - Prob. 158PCh. 7.13 - Prob. 159PCh. 7.13 - Prob. 160PCh. 7.13 - Prob. 161PCh. 7.13 - Prob. 162PCh. 7.13 - Prob. 163PCh. 7.13 - Prob. 164PCh. 7.13 - Prob. 165PCh. 7.13 - The space heating of a facility is accomplished by...Ch. 7.13 - Prob. 167PCh. 7.13 - Prob. 168PCh. 7.13 - Prob. 169RPCh. 7.13 - A refrigerator with a coefficient of performance...Ch. 7.13 - Prob. 171RPCh. 7.13 - Prob. 172RPCh. 7.13 - Prob. 173RPCh. 7.13 - A 100-lbm block of a solid material whose specific...Ch. 7.13 - Prob. 175RPCh. 7.13 - Prob. 176RPCh. 7.13 - A pistoncylinder device initially contains 15 ft3...Ch. 7.13 - Prob. 178RPCh. 7.13 - A 0.8-m3 rigid tank contains carbon dioxide (CO2)...Ch. 7.13 - Helium gas is throttled steadily from 400 kPa and...Ch. 7.13 - Air enters the evaporator section of a window air...Ch. 7.13 - Refrigerant-134a enters a compressor as a...Ch. 7.13 - Prob. 183RPCh. 7.13 - Three kg of helium gas at 100 kPa and 27C are...Ch. 7.13 - Prob. 185RPCh. 7.13 -
7–186 You are to expand a gas adiabatically from...Ch. 7.13 - Prob. 187RPCh. 7.13 - Determine the work input and entropy generation...Ch. 7.13 - Prob. 189RPCh. 7.13 - Prob. 190RPCh. 7.13 - Air enters a two-stage compressor at 100 kPa and...Ch. 7.13 - Steam at 6 MPa and 500C enters a two-stage...Ch. 7.13 - Prob. 193RPCh. 7.13 - Prob. 194RPCh. 7.13 - Prob. 196RPCh. 7.13 - Prob. 197RPCh. 7.13 - 7–198 To control the power output of an isentropic...Ch. 7.13 - Prob. 199RPCh. 7.13 - Prob. 200RPCh. 7.13 - A 5-ft3 rigid tank initially contains...Ch. 7.13 - Prob. 202RPCh. 7.13 - Prob. 203RPCh. 7.13 - Prob. 204RPCh. 7.13 - Prob. 205RPCh. 7.13 - Prob. 206RPCh. 7.13 - Prob. 207RPCh. 7.13 - Prob. 208RPCh. 7.13 - (a) Water flows through a shower head steadily at...Ch. 7.13 - Prob. 211RPCh. 7.13 - Prob. 212RPCh. 7.13 - Prob. 213RPCh. 7.13 - Consider the turbocharger of an internal...Ch. 7.13 - Prob. 215RPCh. 7.13 - Prob. 216RPCh. 7.13 - Prob. 217RPCh. 7.13 - Consider two bodies of identical mass m and...Ch. 7.13 - Prob. 220RPCh. 7.13 - Prob. 222RPCh. 7.13 - Prob. 224RPCh. 7.13 - The polytropic or small stage efficiency of a...Ch. 7.13 - Steam is compressed from 6 MPa and 300C to 10 MPa...Ch. 7.13 - An apple with a mass of 0.12 kg and average...Ch. 7.13 - A pistoncylinder device contains 5 kg of saturated...Ch. 7.13 - Prob. 229FEPCh. 7.13 - Prob. 230FEPCh. 7.13 - A unit mass of a substance undergoes an...Ch. 7.13 - A unit mass of an ideal gas at temperature T...Ch. 7.13 - Prob. 233FEPCh. 7.13 - Prob. 234FEPCh. 7.13 - Air is compressed steadily and adiabatically from...Ch. 7.13 - Argon gas expands in an adiabatic turbine steadily...Ch. 7.13 - Water enters a pump steadily at 100 kPa at a rate...Ch. 7.13 - Air is to be compressed steadily and...Ch. 7.13 - Helium gas enters an adiabatic nozzle steadily at...Ch. 7.13 - Combustion gases with a specific heat ratio of 1.3...Ch. 7.13 - Steam enters an adiabatic turbine steadily at 400C...Ch. 7.13 - Liquid water enters an adiabatic piping system at...Ch. 7.13 - Prob. 243FEPCh. 7.13 - Steam enters an adiabatic turbine at 8 MPa and...Ch. 7.13 - Helium gas is compressed steadily from 90 kPa and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 3. Water enters the constant 125-mm inside-diameter tubes of a boiler at 7.5 MPa and 60°C and leaves the tubes at 6 MPa and 500°C with a velocity of 75 m/s. Calculate the velocity of the water at the tube inlet and the inlet volume flow rate.arrow_forward2. A piston-cylinder device contains 2.4 kg of nitrogen initially at 120 kPa and 27°C. The nitrogen is now compressed slowly in a polytropic process during which PV1.3 = constant until the volume is reduced by one-half. Determine the work done and the heat transfer for this process.arrow_forward1. 1.25 m³ of saturated liquid water at 225°C is expanded isothermally in a closed system until its quality is 75 percent. Determine the total work produced by this expansion, in kJ.arrow_forward
- An undamped single-degree-of-freedom system is subjected to dynamic excitation as shown in Figure 1.• System properties: m = 1, c = 0, k = (6π)2.• Force excitation: p(t) = posin(ωt) where po = 9 and ω = 2π.• Initial conditions: u(t = 0) = 0 and ̇u(t = 0) = 0.Please, complete Parts (a) through (d) using any computational tool of your preference. The preferred toolis MATLAB. Print and turn in a single pdf file that will include your code/calculations and your plots.(a) Generate the solution using a linear interpolation of the load over each time step (note that hereyou can use the undamped coefficients). Plot the displacement response for the first 4 seconds andcompare to the exact closed form solution. Repeat using the following time step sizes, ∆t = 0.01,0.05, 0.15, 0.20 seconds. Include the closed form solution and the solutions for different ∆t values in asingle plot. Please, provide your observations by comparing the closed form solution with the solutionsderived using the four…arrow_forwardAssume multiple single degree of freedom systems with natural periods T ∈ [0.05, 2.00] seconds with in-crement of period dT = 0.05 seconds. Assume three cases of damping ratio: Case (A) ξ = 0%; Case (B)ξ = 2%; Case (C) ξ = 5%. The systems are initially at rest. Thus, the initial conditions are u(t = 0) = 0 anḋu(t = 0) = 0. The systems are subjected to the base acceleration that was provided in the ElCentro.txt file(i.e., first column). For the systems in Case (A), Case (B), and Case (C) and for each natural period computethe peak acceleration, peak velocity, and peak displacement responses to the given base excitation. Please,use the Newmark method for β = 1/4 (average acceleration) to compute the responses. Create threeplots with three lines in each plot. The first plot will have the peak accelerations in y-axis and the naturalperiod of the system in x-axis. The second plot will have the peak velocities in y-axis and the natural periodof the system in x-axis. The third plot will have…arrow_forwardBoth portions of the rod ABC are made of an aluminum for which E = 70 GPa. Based on the given information find: 1- deformation at A 2- stress in BC 3- Total strain 4- If v (Poisson ratio is 0.25, find the lateral deformation of AB Last 3 student ID+ 300 mm=L2 724 A P=Last 2 student ID+ 300 KN 24 24 Diameter Last 2 student ID+ 15 mm Last 3 student ID+ 500 mm=L1 724 C B 24 Q=Last 2 student ID+ 100 KN 24 Diameter Last 2 student ID+ 40 mmarrow_forward
- Q2Two wooden members of uniform cross section are joined by the simple scarf splice shown. Knowing that the maximum allowable tensile stress in the glued splice is 75 psi, determine (a) the largest load P that can be safely supported, (b) the corresponding shearing stress in the splice. น Last 1 student ID+5 inch=W =9 4 L=Last 1 student ID+8 inch =12 60° P'arrow_forwardQ4 The two solid shafts are connected by gears as shown and are made of a steel for which the allowable shearing stress is 7000 psi. Knowing the diameters of the two shafts are, respectively, dBC determine the largest torque Tc that can be applied at C. 4 and dEF dBC=Last 1 student ID+3 inch dEF=Last 1 student ID+1 inch 7 R=Last 1 Student ID+5 inch 9 R B Tc 2.5 in. E TF Harrow_forwardExperiment تكنولوجيا السيارات - Internal Forced convenction Heat transfer Air Flow through Rectangular Duct. objective: Study the convection heat transfer of air flow through rectangular duct. Valve Th Top Dead Centre Exhaust Valve Class CP. N; ~ RIVavg Ti K 2.11 Te To 18.8 21.3 45.8 Nath Ne Pre Calculations:. Q = m cp (Te-Ti) m: Varg Ac Acca*b Q=hexp As (Ts-Tm) 2 2.61 18.5 20.846.3 Tm = Te-Ti = 25 AS-PL = (a+b)*2*L Nu exp= Re-Vavy D heep Dh k 2ab a+b Nu Dh the- (TS-Tm) Ts. Tmy Name / Nu exp Naxe بب ارتدان العشريarrow_forward
- Procedure:1- Cartesian system, 2D3D,type of support2- Free body diagram3 - Find the support reactions4- If you find a negativenumber then flip the force5- Find the internal force3D∑Fx=0∑Fy=0∑Fz=0∑Mx=0∑My=0\Sigma Mz=02D\Sigma Fx=0\Sigma Fy=0\Sigma Mz=05- Use method of sectionand cut the elementwhere you want to findarrow_forwardProcedure:1- Cartesian system, 2D3D,type of support2- Free body diagram3 - Find the support reactions4- If you find a negativenumber then flip the force5- Find the internal force3D∑Fx=0∑Fy=0∑Fz=0∑Mx=0∑My=0\Sigma Mz=02D\Sigma Fx=0\Sigma Fy=0\Sigma Mz=05- Use method of sectionand cut the elementwhere you want to findthe internal force andkeep either side of thearrow_forwardProcedure: 1- Cartesian system, 2D3D, type of support 2- Free body diagram 3 - Find the support reactions 4- If you find a negative number then flip the force 5- Find the internal force 3D ∑Fx=0 ∑Fy=0 ∑Fz=0 ∑Mx=0 ∑My=0 ΣMz=0 2D ΣFx=0 ΣFy=0 ΣMz=0 5- Use method of section and cut the element where you want to find the internal force and keep either side of thearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Power Plant Explained | Working Principles; Author: RealPars;https://www.youtube.com/watch?v=HGVDu1z5YQ8;License: Standard YouTube License, CC-BY