
ORGANIC CHEMISTRY (LOOSELEAF)
6th Edition
ISBN: 9781260475630
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.1, Problem 1P
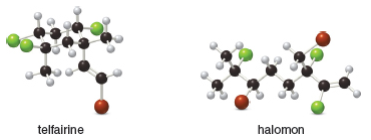
Telfairine, a naturally occurring insecticide, and halomon, an antitumor agent, are two
polyhalogenated compounds isolated from red algae. (a) Classify each halide bonded to an
hybridized carbon as 1°, 2°, or 3°. (b) Label each halide as vinyl, allylic, or neither.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
ол
2.
восцапан
(46:00)
Curtius rearrangment
1. NaN3, heat
-OH
Question 1. Please predict the products for each of the following reactions.
Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both).
If a mixture of enantiomers is formed, please draw all the enantiomers.
Electrochemistry. Briefly describe the Donnan potential.
Chapter 7 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)
Ch. 7.1 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7.2 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7.2 - Prob. 3PCh. 7.3 - An sp3 hybridized CCl bond is more polar than an...Ch. 7.4 - Prob. 5PCh. 7.6 - Prob. 6PCh. 7.7 - Prob. 10PCh. 7.8 - Prob. 15PCh. 7.8 - Prob. 16PCh. 7.8 - Prob. 17P
Ch. 7.11 - Prob. 18PCh. 7.11 - Prob. 19PCh. 7.11 - Draw the product of each SN2 reaction and indicate...Ch. 7.11 - Prob. 21PCh. 7.11 - Prob. 22PCh. 7.12 - What happens to the rate of an SN1 reaction under...Ch. 7.12 - Draw the products of each SN1 reaction and...Ch. 7.13 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7.15 - Problem 7.30 For each alkyl halide and...Ch. 7.15 - Prob. 30PCh. 7.15 - Prob. 31PCh. 7.15 - Prob. 32PCh. 7.15 - Prob. 33PCh. 7.15 - Prob. 34PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 69PCh. 7 - Prob. 70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 72PCh. 7 - Prob. 78P
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. A gene is a segment of DNA that has the information to produce a functional product. The functional product ...
Genetics: Analysis and Principles
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate what the Luther equation is used for?arrow_forwardIndicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forward
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forward
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License