
Concept explainers
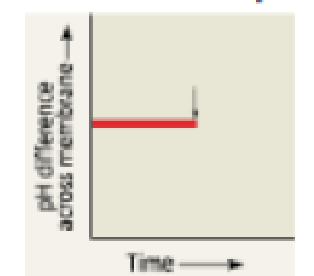
DRAW IT The graph here shows the pH difference across the inner mitochondrial membrane over time in an actively respiring cell. At the time indicated by the vertical arrow, a
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Campbell Biology in Focus, Books a la Carte Edition; Modified Mastering Biology with Pearson eText - ValuePack Access Card - for Campbell Biology in Focus (2nd Edition)
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Laboratory Manual For Human Anatomy & Physiology
SEELEY'S ANATOMY+PHYSIOLOGY
Physical Universe
Biology: Life on Earth with Physiology (11th Edition)
Cosmic Perspective Fundamentals
- View History Bookmarks Window Help Quarter cements ents ons (17) YouTube Which amino acids would you expect to find marked on the alpha helix? canvas.ucsc.edu ucsc Complaint and Grievance Process - Academic Personnel pach orations | | | | | | | | | | | | | | | | 000000 000000000 | | | | | | | | | | | | | | | | | | | | | | 00000000 scope vious De 48 12.415 KATPM FEB 3 F1 F2 80 F3 a F4 F5 2 # 3 $ 85 % tv N A の Mon Feb 3 10:24 PM Lipid bilayer Submit Assignment Next > ZOOM < Å DII 8 བ བ F6 16 F7 F8 F9 F10 34 F11 F12 & * ( 6 7 8 9 0 + 11 WERTY U { 0 } P deletearrow_forwardDifferent species or organisms research for ecologyarrow_forwardWhat is the result of the following gram stain: positive ○ capsulated ○ acid-fast ○ negativearrow_forward
- What type of stain is the image below: capsule stain endospore stain gram stain negative stain ASM MicrobeLibrary.org Keplingerarrow_forwardWhat is the result of the acid-fast stain below: Stock Images by Getty Images by Getty Images by Getty Images by Getty Image Getty Images St Soy Getty Images by Getty Images by Getty Images Joy Getty encapsulated O endosporulating negative ○ positivearrow_forwardYou have a stock vial of diligence 75mg in 3ml and need to draw up a dose of 50mg for your patient.how many mls should you draw up to give this dosearrow_forward
- You are recquired to administer 150mg hydrocortisone intravenously,how many mls should you give?(stock =hydrocortisone 100mg in 2mls)arrow_forwardIf someone was working with a 50 MBq F-18 source, what would be the internal and external dose consequences?arrow_forwardWe will be starting a group project next week where you and your group will research and ultimately present on a current research article related to the biology of a pathogen that infects humans. The article could be about the pathogen itself, the disease process related to the pathogen, the immune response to the pathogen, vaccines or treatments that affect the pathogen, or other biology-related study about the pathogen. I recommend that you choose a pathogen that is currently interesting to researchers, so that you will be able to find plenty of articles about it. Avoid choosing a historical disease that no longer circulates. List 3 possible pathogens or diseases that you might want to do for your group project.arrow_forward
- not use ai pleasearrow_forwardDNK dagi nukleotidlar va undan sintezlangan oqsildagi peptid boglar farqi 901 taga teng bo'lib undagi A jami H boglardan 6,5 marta kam bo'lsa DNK dagi jami H bog‘lar sonini topingarrow_forwardOne of the ways for a cell to generate ATP is through the oxidative phosphorylation. In oxidative phosphorylation 3 ATP are produced from every one NADH molecule. In respiration, every glucose molecule produces 10 NADH molecules. If a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning





