
Chemistry Atoms First, Second Edition
2nd Edition
ISBN: 9781308211657
Author: Burdge
Publisher: McGraw Hill
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.92QP
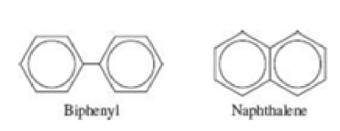
Determine which of these molecules has a more delocalized orbital, and justify your choice. (Hint: Both molecules contain two benzene rings. In naphthalene, the two rings are fused together. In biphenyl, the two rings are joined by a single bond around which the two rings can rotate.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
cold
KMnO4, NaOH
2. DMS
1. 03
CH3OH
Br2
1.
03
2. (CH3)2S
H₂
Pd or Pt (catalyst)
HBr
18
19
20 1
HBr
ROOR (peroxide)
H₂O
H₂SO4
HCI
HI
17
16
6
15
MCPBA
1. BH3 THF
2. H₂O2, NaOH
1. OsO4
2. H₂O₂
110
CH3CO₂H
(peroxyacid)
1. MCPBA
2. H₂O*
Br2
H₂O
BH3 THF
B12
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
Bra
A
B
C
D
H
OH
H
OH
OH
H
OH
α α α
OH
H
OH
OH
фон
d
H
"H
Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.
Electrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.
Chapter 7 Solutions
Chemistry Atoms First, Second Edition
Ch. 7.1 - Determine the shapes of (a) SO3 and (b) ICl4.Ch. 7.1 - Determine the shapes of (a) CO2 and (b) SCl2.Ch. 7.1 - (a) From what group must the terminal atoms come...Ch. 7.1 - These four models may represent molecules or...Ch. 7.1 - Acetic acid, the substance that gives vinegar its...Ch. 7.1 - Ethanolamine (HOCH2CH2NH2) has a smell similar to...Ch. 7.1 - The bond angle in NH3 is significantly smaller...Ch. 7.1 - Which of these models represents a species in...Ch. 7.1 - Prob. 7.1.1SRCh. 7.1 - Prob. 7.1.2SR
Ch. 7.1 - Prob. 7.1.3SRCh. 7.1 - Prob. 7.1.4SRCh. 7.2 - Prob. 7.3WECh. 7.2 - Prob. 3PPACh. 7.2 - For each of the following hypothetical molecules,...Ch. 7.2 - Which of these models could represent a polar...Ch. 7.2 - Prob. 7.2.1SRCh. 7.2 - Prob. 7.2.2SRCh. 7.3 - Prob. 7.4WECh. 7.3 - Prob. 4PPACh. 7.3 - Prob. 4PPBCh. 7.3 - Prob. 4PPCCh. 7.3 - Prob. 7.3.1SRCh. 7.3 - Prob. 7.3.2SRCh. 7.4 - Hydrogen selenide (H2Se) is a foul-smelling gas...Ch. 7.4 - Prob. 5PPACh. 7.4 - For which molecule(s) can we not use valence bond...Ch. 7.4 - Which of these models could represent a species...Ch. 7.4 - Prob. 7.4.1SRCh. 7.4 - Prob. 7.4.2SRCh. 7.5 - Prob. 7.6WECh. 7.5 - Use hybrid orbital theory to describe the bonding...Ch. 7.5 - Prob. 6PPBCh. 7.5 - Prob. 6PPCCh. 7.5 - Prob. 7.5.1SRCh. 7.5 - Prob. 7.5.2SRCh. 7.6 - Thalidomide (C13H10N2O4) is a sedative and...Ch. 7.6 - The active ingredient in Tylenol and a host of...Ch. 7.6 - Determine the total number of sigma and pi bonds...Ch. 7.6 - In terms of valence bond theory and hybrid...Ch. 7.6 - In addition to its rise in aqueous solution as a...Ch. 7.6 - Use valence bond theory and hybrid orbitals to...Ch. 7.6 - Use valence bond theory and hybrid orbitals to...Ch. 7.6 - Explain why hybrid orbitals are necessary to...Ch. 7.6 - Prob. 7.6.1SRCh. 7.6 - Prob. 7.6.2SRCh. 7.6 - Prob. 7.6.3SRCh. 7.6 - Prob. 7.6.4SRCh. 7.7 - Prob. 7.9WECh. 7.7 - Use molecular orbital theory to determine whether...Ch. 7.7 - Use molecular orbital theory to determine whether...Ch. 7.7 - For most of the homonuclear diatomic species shown...Ch. 7.7 - Prob. 7.7.1SRCh. 7.7 - Prob. 7.7.2SRCh. 7.7 - Prob. 7.7.3SRCh. 7.7 - Prob. 7.7.4SRCh. 7.8 - It takes three resonance structures to represent...Ch. 7.8 - Use a combination of valence bond theory and...Ch. 7.8 - Use a combination of valence bond theory and...Ch. 7.8 - Prob. 10PPCCh. 7.8 - Prob. 7.8.1SRCh. 7.8 - Prob. 7.8.2SRCh. 7.8 - Prob. 7.8.3SRCh. 7.8 - Prob. 7.8.4SRCh. 7 - Prob. 7.1QPCh. 7 - Sketch the shape of a linear triatomic molecule, a...Ch. 7 - Prob. 7.3QPCh. 7 - Prob. 7.4QPCh. 7 - In the trigonal bipyramidal arrangement, why does...Ch. 7 - Prob. 7.6QPCh. 7 - Predict the geometry of the following molecules...Ch. 7 - Prob. 7.8QPCh. 7 - Predict the geometries of the following species...Ch. 7 - Predict the geometries of the following ions: (a)...Ch. 7 - Prob. 7.11QPCh. 7 - Prob. 7.12QPCh. 7 - Prob. 7.13QPCh. 7 - Describe the geometry about each of the central...Ch. 7 - Prob. 7.15QPCh. 7 - Prob. 7.16QPCh. 7 - Prob. 7.17QPCh. 7 - Prob. 7.18QPCh. 7 - Prob. 7.19QPCh. 7 - Prob. 7.20QPCh. 7 - Prob. 7.21QPCh. 7 - Prob. 7.22QPCh. 7 - Explain the term polarizability. What kind of...Ch. 7 - Prob. 7.24QPCh. 7 - What physical properties are determined by the...Ch. 7 - Prob. 7.26QPCh. 7 - Describe the types of intermolecular forces that...Ch. 7 - The compounds Br2 and ICl are isoelectronic (have...Ch. 7 - If you lived in Alaska, which of the following...Ch. 7 - The binary hydrogen compounds of the Group 4A...Ch. 7 - List the types of intermolecular forces that exist...Ch. 7 - Prob. 7.32QPCh. 7 - Prob. 7.33QPCh. 7 - Prob. 7.34QPCh. 7 - Diethyl ether has a boiling point of 34.5C, and...Ch. 7 - Prob. 7.36QPCh. 7 - Which substance in each of the following pairs...Ch. 7 - Prob. 7.38QPCh. 7 - What kind of attractive forces must be overcome to...Ch. 7 - Prob. 7.40QPCh. 7 - Prob. 7.41QPCh. 7 - The following compounds have the same molecular...Ch. 7 - Prob. 7.43QPCh. 7 - Prob. 7.44QPCh. 7 - Use valence bond theory to explain the bonding in...Ch. 7 - Prob. 7.46QPCh. 7 - Prob. 7.47QPCh. 7 - Prob. 7.48QPCh. 7 - Prob. 7.49QPCh. 7 - What is the hybridization of atomic orbitals? Why...Ch. 7 - Prob. 7.51QPCh. 7 - Prob. 7.52QPCh. 7 - Prob. 7.53QPCh. 7 - Describe the bonding scheme of the AsH3 molecule...Ch. 7 - Prob. 7.55QPCh. 7 - Prob. 7.56QPCh. 7 - Describe the hybridization of phosphorus in PF5.Ch. 7 - Prob. 7.58QPCh. 7 - Prob. 7.59QPCh. 7 - Prob. 7.1VCCh. 7 - Prob. 7.2VCCh. 7 - Prob. 7.3VCCh. 7 - Prob. 7.4VCCh. 7 - Prob. 7.60QPCh. 7 - Which of the following pairs of atomic orbitals of...Ch. 7 - Prob. 7.62QPCh. 7 - Prob. 7.63QPCh. 7 - Prob. 7.64QPCh. 7 - Prob. 7.65QPCh. 7 - Prob. 7.66QPCh. 7 - Prob. 7.67QPCh. 7 - Prob. 7.68QPCh. 7 - Benzo[a]pyrene is a potent carcinogen found in...Ch. 7 - What is molecular orbital theory? How does it...Ch. 7 - Define the following terms: bonding molecular...Ch. 7 - Prob. 7.72QPCh. 7 - Prob. 7.73QPCh. 7 - Prob. 7.74QPCh. 7 - Prob. 7.75QPCh. 7 - Draw a molecular orbital energy level diagram for...Ch. 7 - Prob. 7.77QPCh. 7 - Prob. 7.78QPCh. 7 - Prob. 7.79QPCh. 7 - Acetylene (C2H2) has a tendency to lose two...Ch. 7 - Compare the Lewis and molecular orbital treatments...Ch. 7 - Prob. 7.82QPCh. 7 - Prob. 7.83QPCh. 7 - Prob. 7.84QPCh. 7 - Prob. 7.85QPCh. 7 - Draw the molecular orbital diagram for the cyanide...Ch. 7 - Given that BeO is diamagnetic, use a molecular...Ch. 7 - Prob. 7.88QPCh. 7 - Prob. 7.89QPCh. 7 - Both ethylene (C2H4) and benzene (C6H6) contain...Ch. 7 - Chemists often represent benzene with the...Ch. 7 - Determine which of these molecules has a more...Ch. 7 - Nitryl fluoride (FNO2) is used in rocket...Ch. 7 - Describe the bonding in the nitrate ion NO3 in...Ch. 7 - Prob. 7.95QPCh. 7 - Prob. 7.96QPCh. 7 - Prob. 7.97QPCh. 7 - Prob. 7.98QPCh. 7 - Prob. 7.99QPCh. 7 - Antimony pentafluoride (SbF5) combines with XeF4...Ch. 7 - Prob. 7.101QPCh. 7 - The molecular model of nicotine (a stimulant) is...Ch. 7 - Predict the bond angles for the following...Ch. 7 - The germanium pentafluoride anion (GeF5) has been...Ch. 7 - Draw Lewis structures and give the other...Ch. 7 - Which figure best illustrates the hybridization of...Ch. 7 - Prob. 7.107QPCh. 7 - Prob. 7.108QPCh. 7 - Prob. 7.109QPCh. 7 - Prob. 7.110QPCh. 7 - Prob. 7.111QPCh. 7 - Cyclopropane (C3H6) has the shape of a triangle in...Ch. 7 - The compound 1,2-dichloroethane (C2H4Cl2) is...Ch. 7 - Prob. 7.114QPCh. 7 - Prob. 7.115QPCh. 7 - Prob. 7.116QPCh. 7 - Prob. 7.117QPCh. 7 - Prob. 7.118QPCh. 7 - The amino acid selenocysteine is one of the...Ch. 7 - Prob. 7.120QPCh. 7 - Prob. 7.121QPCh. 7 - Prob. 7.122QPCh. 7 - Gaseous or highly volatile liquid anesthetics are...Ch. 7 - Prob. 7.124QPCh. 7 - Prob. 7.125QPCh. 7 - Two of the drugs that are prescribed for the...Ch. 7 - Prob. 7.127QPCh. 7 - Prob. 7.128QPCh. 7 - The BO+ ion is paramagnetic. Determine (a) whether...Ch. 7 - Use molecular orbital theory to explain the...Ch. 7 - Which best illustrates the change in geometry...Ch. 7 - Prob. 7.132QPCh. 7 - Prob. 7.133QPCh. 7 - Aluminum trichloride (AlCl3) is an...Ch. 7 - Prob. 7.135QPCh. 7 - Prob. 7.136QPCh. 7 - Prob. 7.137QPCh. 7 - Consider an N2 molecule in its first excited...Ch. 7 - The Lewis structure for O2 is Use molecular...Ch. 7 - Draw the Lewis structure of ketene (C2H2O) and...Ch. 7 - The compound TCDD, or...Ch. 7 - Name the kinds of attractive forces that must be...Ch. 7 - Carbon monoxide (CO) is a poisonous compound due...Ch. 7 - Prob. 7.144QPCh. 7 - Prob. 7.145QPCh. 7 - Prob. 7.146QPCh. 7 - Prob. 7.147QPCh. 7 - Prob. 7.148QPCh. 7 - Prob. 7.1KSPCh. 7 - Which of the following species does not have...Ch. 7 - Prob. 7.3KSPCh. 7 - Prob. 7.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forwardGiven the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY