
Concept explainers
a.
Interpretation:
Whether
Concept Introduction:
We can predict the shape of a particular molecule by the knowledge of their
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
a.
Answer to Problem 7.67PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of Xeis
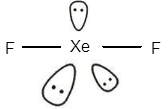
The geometry of
The central atom has three lone pairs of electrons
b.
Interpretation:
Whether
Concept Introduction:
We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
b.
Explanation of Solution
The geometry of
is linear and it has 0 lone pairs of an electron on the central atom The electronic configuration of C is
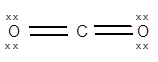
The geometry of
The central atom has no lone pair of electrons
c.
Interpretation:
Whether
Concept Introduction We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
c.
Answer to Problem 7.67PAE
lp-lp>lp-bp>bp-bp
Solution: The geometry of
Explanation of Solution
The electronic configuration of Be is
Structure of
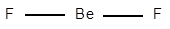
The geometry of
The central atom has no lone pair of electrons
d.
Interpretation:
Whether
Concept Introduction We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
d.
Answer to Problem 7.67PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of O is
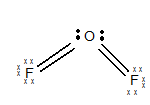
The geometry of
The central atom has two lone pairs of electrons
Want to see more full solutions like this?
Chapter 7 Solutions
Bundle: Chemistry for Engineering Students, Loose-Leaf Version, 4th + OWLv2 with MindTap Reader with Student Solutions Manual, 1 term (6 months) Printed Access Card
- Michael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forward
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

