
(a)
Interpretation:
The electron-rich sites and electron-poor sites in the given elementary steps are to be identified.
Concept introduction:
An atom with partial or full negative charge is an electron-rich site whereas an atom with partial or full positive charge is an electron-poor site. In an elementary step, electrons tend to flow from an electron-rich site to an electron-poor site.
Answer to Problem 7.54P
The electron-rich and electron-poor sites for first elementary step are:
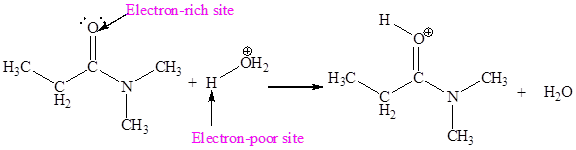
The electron-rich and electron-poor sites for second elementary step are:
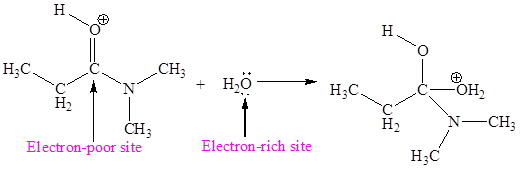
The electron-rich and electron-poor sites for third elementary step are:
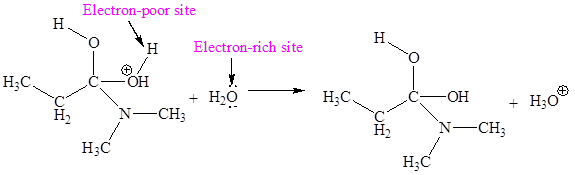
The electron-rich and electron-poor sites for fourth elementary step are:
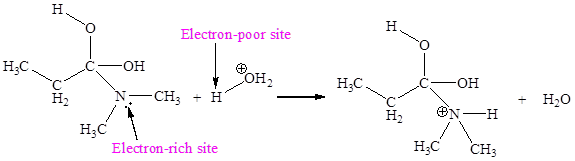
The electron-rich and electron-poor sites for fifth elementary step are:
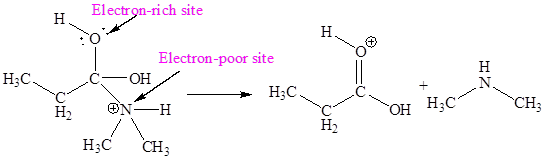
The electron-rich and electron-poor sites for sixth elementary step are:
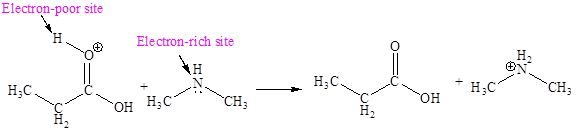
Explanation of Solution
The first elementary step is:
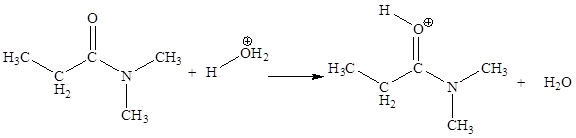
In the given elementary step, on the reactant side the oxygen atom which is a part carbonyl group having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
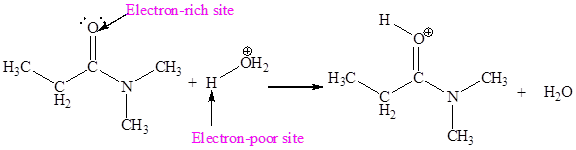
The second elementary step is:
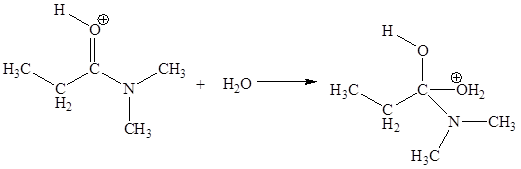
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The carbon atom adjacent to positively charged oxygen is electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
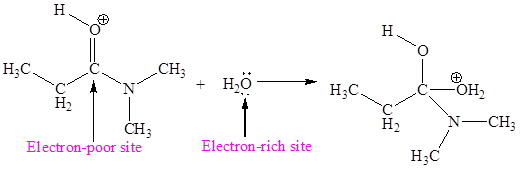
The third elementary step is:
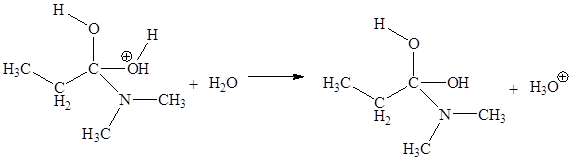
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The H atom adjacent to positively charged oxygen is electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
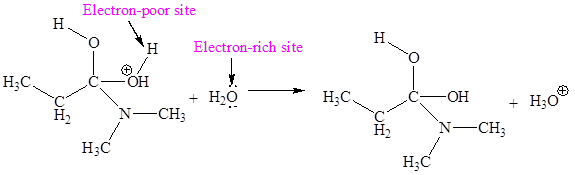
The fourth elementary step is:
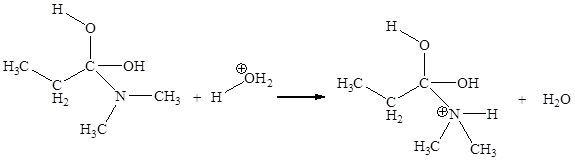
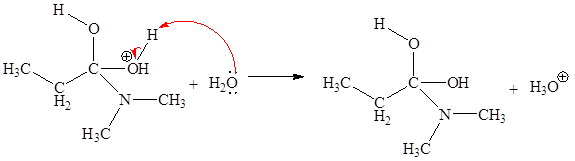
In the given elementary step, on the reactant side the nitrogen atom having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
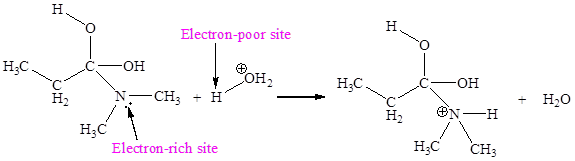
The fifth elementary step is:
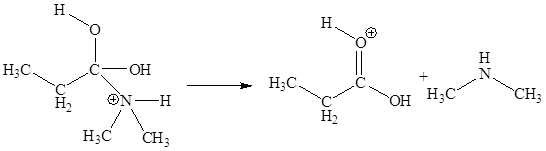
In the given elementary step, on the reactant side the oxygen atom of C-O bond having lone pairs is the electron-rich site. The nitrogen atom which is positively charged is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
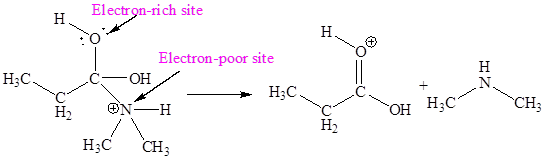
The sixth elementary step is:
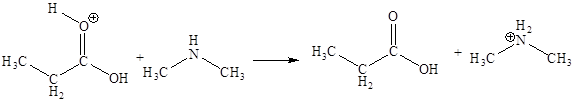
In the given elementary step, on the reactant side the hydrogen atom adjacent to positively charged oxygen atom is the electron-poor site. The nitrogen atom which is having lone pair of electrons is the electron-rich site. The electron-rich and electron-poor sites for this step are labeled below:
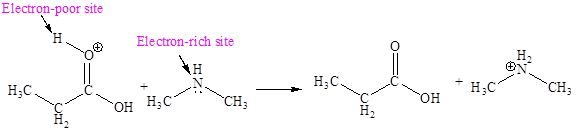
The electron-rich site and the electron-poor sites in each elementary step are identified on the basis of the negative and partial positive charge on respective atoms.
(b)
Interpretation:
In each of the given elementary steps the appropriate curved arrows are to be drawn.
Concept introduction:
The curved arrow can draw from electron rich site to an electron poor site to show the flow of electron from electron-rich site to electron-poor site. The first curved arrow drawn from the lone pair of negatively charged atom of electron-rich site to the less electronegative atom of electron-poor site. The second curved arrow drawn from the region between the less electronegative atom and more electronegative atom towards the more electronegative atom indicating the breaking of bond.
Answer to Problem 7.54P
The curved arrow mechanism for the first step is:
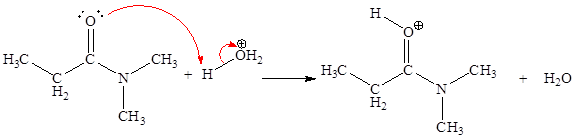
The curved arrow mechanism for the second step is:
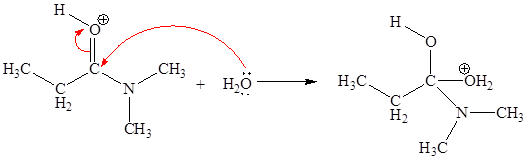
The curved arrow mechanism for the third step is:
The curved arrow mechanism for the fourth step is:
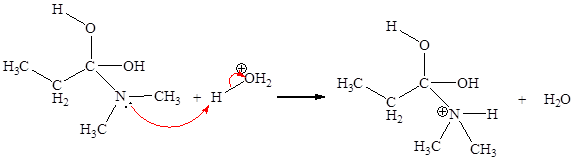
The curved arrow mechanism for the fifth step is:
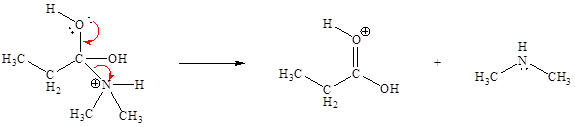
The curved arrow mechanism for the sixth step is:
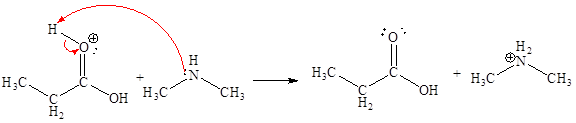
Explanation of Solution
The first elementary step is:
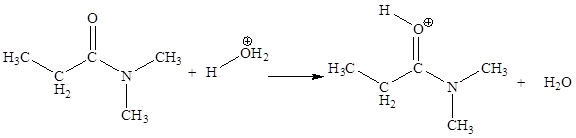
In above step the oxygen is an electron-rich site which attack at hydrogen atom bonded to positively charged oxygen atom is an electron poor site.
The curved arrow mechanism for this step is shown below:
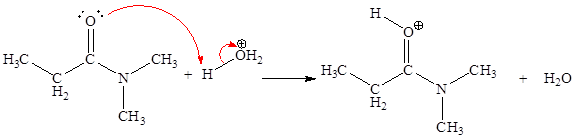
The first curved arrow is drawn from the lone pair of electron-rich oxygen to the electron-poor hydrogen atom representing the formation of
The second elementary step is:
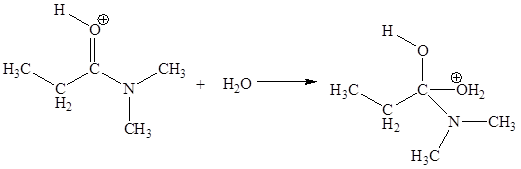
In this elementary step, the oxygen atom of water molecule having lone pair is the electron-rich site and the carbon atom adjacent to positively charged oxygen atom is electron-poor site. The curved arrow mechanism for this step is shown below:
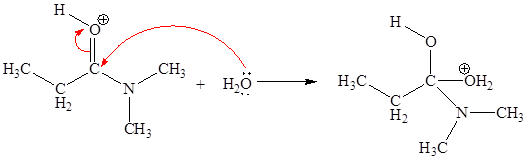
The first curved arrow is drawn from the lone pair of electron-rich oxygen atom to the electron-poor carbon atom representing the formation of new
The third elementary step is:
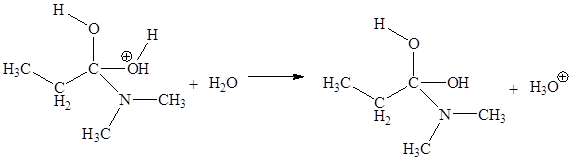
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is an electron-poor site. The curved arrow mechanism for this step is shown below:

The first curved arrow is drawn from the lone pair of electron-rich oxygen atom of water to the electron-poor hydrogen atom representing the formation of new
The fourth elementary step is:
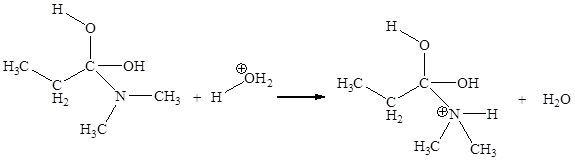
In the given elementary step, on the reactant side the nitrogen atom having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The curved arrow mechanism for this step is shown below:
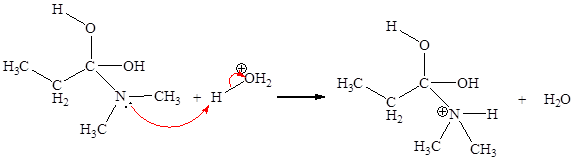
The first curved arrow is drawn from the lone pair of electron-rich nitrogen to the electron-poor hydrogen atom representing the formation of
The fifth elementary step is:
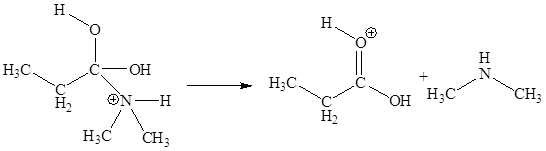
In the given elementary step, on the reactant side the oxygen atom of C-O bond having lone pairs is the electron-rich site. The nitrogen atom which is positively charged is the electron-poor site. The curved arrow mechanism for this step is shown below: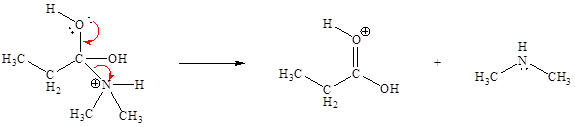
The first curved arrow is drawn from the lone pair of electron-rich oxygen to the
The sixth elementary step is:
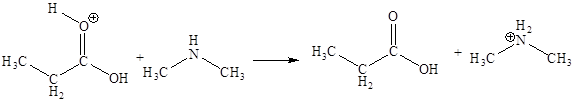
In the given elementary step, on the reactant side the hydrogen atom adjacent to positively charged oxygen atom is the electron-poor site. The nitrogen atom which is having lone pair of electrons is the electron-rich site. The curved arrow mechanism for this step is shown below:
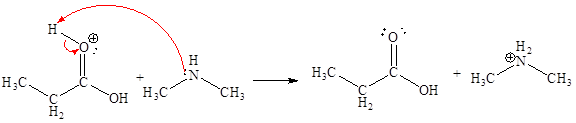
The first curved arrow is drawn from the lone pair of electron-rich nitrogen to the electron-poor hydrogen atom representing the formation of
The curved arrows for each of the given elementary steps are drawn from electron rich site to electron poor site and the less electronegative atom to more electronegative atom representing the formation and breaking of respective bonds.
(c)
Interpretation:
The names of each elementary step are to be identified.
Concept introduction:
In the nucleophilic addition step, the nucleophile forms a bond to the less electronegative atom and the
In a nucleophilic elimination step, a lone pair of electrons from a more electronegative atom forms a π bond to a less electronegative atom. A leaving group is simultaneously expelled to avoid exceeding an octet on the less electronegative atom.
An elementary step in which a proton is transferred from electron-poor site to electron- rich site and one bond is broken and another is formed simultaneously is called proton transfer step.
Answer to Problem 7.54P
The first elementary step is proton transfer reaction.
The nucleophilic addition reaction is the second elementary step.
The third elementary step is proton transfer reaction.
The fourth elementary step is proton transfer reaction.
The fifth elementary step is nucleophilic elimination reaction.
The sixth elementary step is proton transfer reaction.
Explanation of Solution
The first elementary step is:
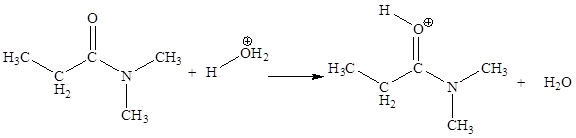
In the above elementary step, proton transferred from the positively charged oxygen of water molecule to the electron-rich oxygen atom. In this step, one
The second elementary step is:
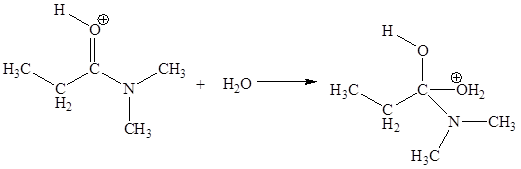
In the above elementary step, the oxygen atom of water forms a bond to the less electronegative carbon atom and the
The third elementary step is:
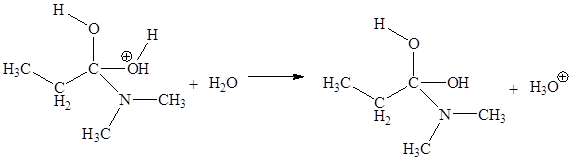
In the above elementary step, proton transferred from the positively charged oxygen of to the electron-rich oxygen atom of water molecule. In this step, one
The fourth elementary step is:
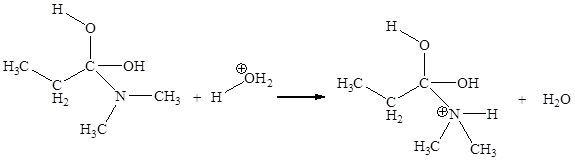
In the above elementary step, proton transferred from the positively charged oxygen of water molecule to the electron-rich nitrogen atom. In this step, one
The fifth elementary step is:
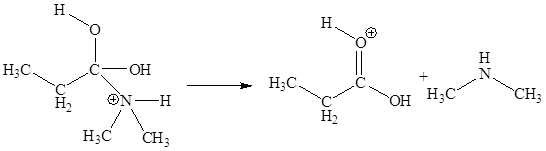
In the above elementary step, a lone pair of electrons from oxygen atom forms a π bond to a less electronegative carbon atom. A leaving group is simultaneously expelled to avoid exceeding an octet on the less electronegative carbon atom. Therefore, the step is named as nucleophilic elimination step.
The sixth elementary step is:
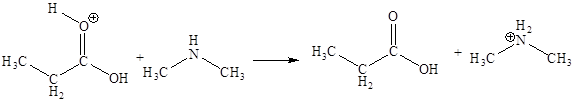
In the above elementary step, proton transferred from the positively charged oxygen of to the electron-rich nitrogen atom. In this step, one
The names for the given elementary steps are identified on the basis of type of bond forming and breaking.
Want to see more full solutions like this?
Chapter 7 Solutions
Get Ready for Organic Chemistry
- Find one pertinent analytical procedure for each of following questions relating to food safety analysis. Question 1: The presence of lead, mercury and cadmium in canned tuna Question 2: Correct use of food labellingarrow_forwardFormulate TWO key questions that are are specifically in relation to food safety. In addition to this, convert these questions into a requirement for chemical analysis.arrow_forwardWhat are the retrosynthesis and forward synthesis of these reactions?arrow_forward
- Which of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forwardWhat is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forwardThe reaction of what nucleophile and substrate is represented by the following transition state? CH3 CH3O -Br อ δ CH3 Methanol with 2-bromopropane Methanol with 1-bromopropane Methoxide with 1-bromopropane Methoxide with 2-bromopropanearrow_forward
- What is the stepwise mechanism for this reaction?arrow_forward32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forward
- K for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forwardDraw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





