
Concept explainers
(a)
Interpretation:
The electron-rich sites and electron-poor sites in the given elementary steps are to be identified.
Concept introduction:
An atom with a partial or full negative charge is an electron-rich site whereas an atom with a partial or full positive charge is an electron-poor site. In an elementary step, electrons tend to flow from an electron-rich site to an electron-poor site.
Answer to Problem 7.53P
The electron-rich and electron-poor sites for each elementary step are:
First elementary step:
Second elementary step:
Third elementary step:
Fourth elementary step:
Fifth elementary step:
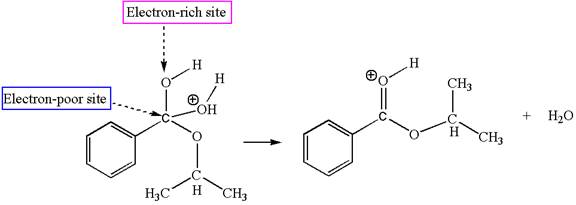
Sixth elementary step:
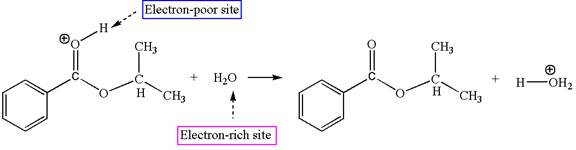
Explanation of Solution
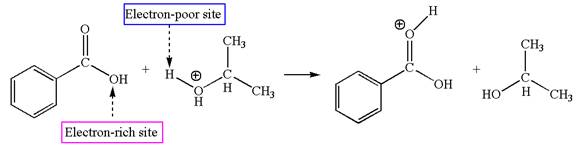
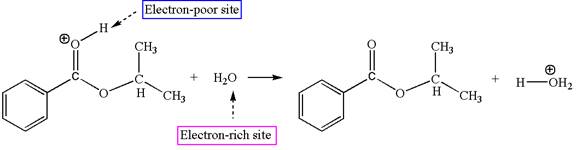
The first elementary step is:
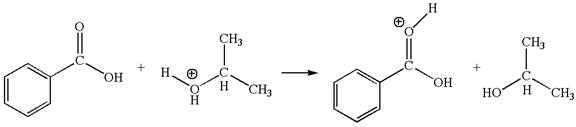
In this step, the carbonyl oxygen of benzoic acid having lone pairs is the electron-rich site. The hydrogen atom carry partial positive charge due to adjacent positively charged oxygen atom and this is an electron-poor site. The electron-rich and electron-poor sites for this step are labelled below:
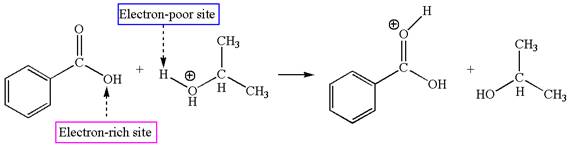
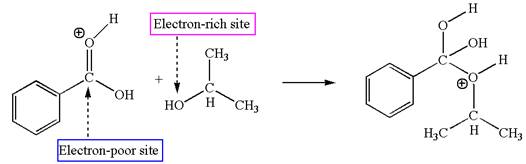
The second elementary step is:
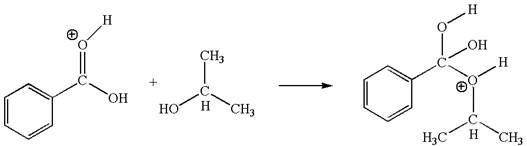
In this step, the hydroxyl oxygen of isopropanol having lone pair is the electron-rich site. The carbonyl carbon carries a partial positive charge due to polar
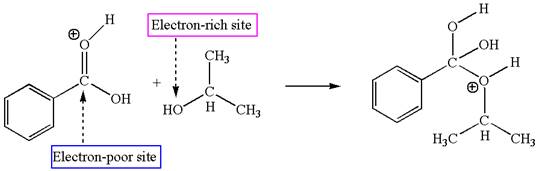
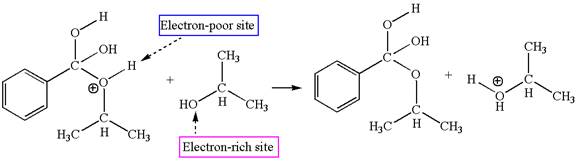
The third elementary step is:
In this step, the hydroxyl oxygen of isopropannol having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labelled below:
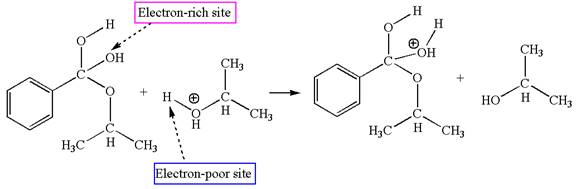
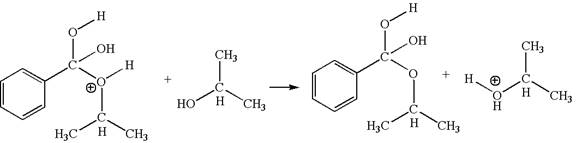
The fourth elementary step is:
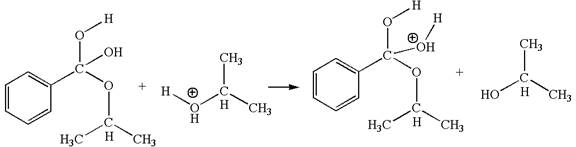
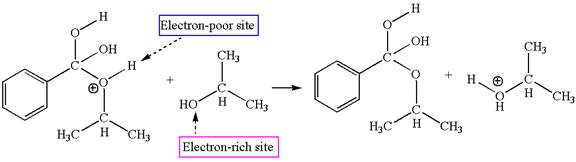
In this step, the hydroxyl groups bonded to the carbon having benzene ring attached are identical. One of the hydroxyl oxygen having lone pairs is the electron-rich site. The hydrogen atom carry partial positive charge due to adjacent positively charged oxygen atom and this is an electron-poor site. The electron-rich and electron-poor sites for this step are labelled below:
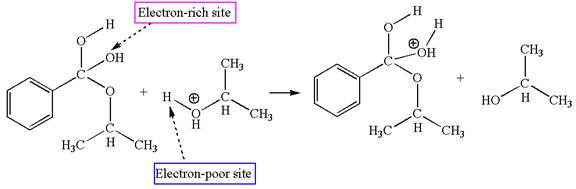
The fifth elementary step is:
In this step, the hydroxyl oxygen having lone pairs is the electron-rich site. The carbon bonded to three oxygen atoms has partial positive charge due to electronegativity of oxygen atoms is the electron-poor site. The electron-rich and electron-poor sites for this step are labelled below:
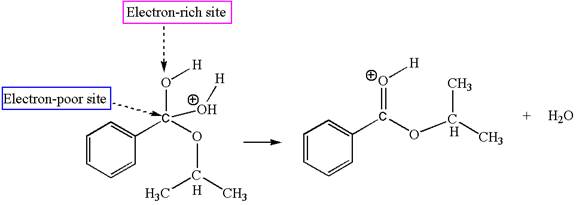
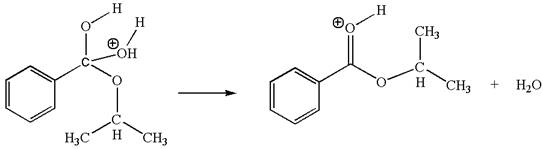
The sixth elementary step is:
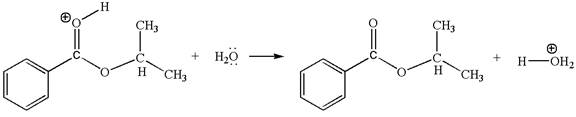
In this step, the oxygen atom of a water molecule having lone pairs is an electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labelled below:
The electron-rich site and the electron-poor sites in each elementary step are identified on the basis of the negative and partial positive charge on respective atoms.
(b)
Interpretation:
In each of the given elementary steps, the appropriate curved arrows are to be drawn.
Concept introduction:
The curved arrow can be drawn from electron-rich site to an electron-poor site to show the flow of electron from electron-rich site to electron-poor site. The first curved arrow is drawn from the lone pair of negatively charged atom of electron-rich site to the less electronegative atom of electron-poor site. The second curved arrow is drawn from the region between the less electronegative atom and more electronegative atom towards the more electronegative atom indicating the breaking of bond.
Answer to Problem 7.53P
The curved arrow mechanism for each elementary steps are:
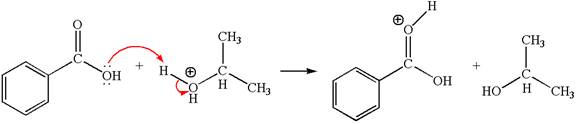
The first elementary step:
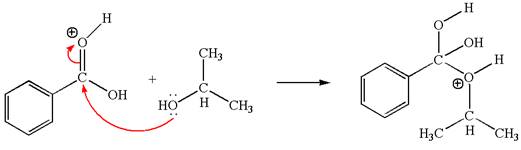
The second elementary step:
The third elementary step:
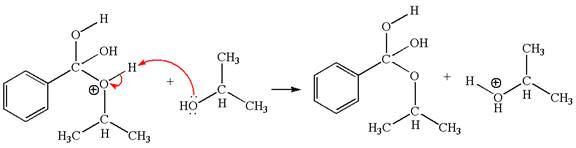
The fourth elementary step:
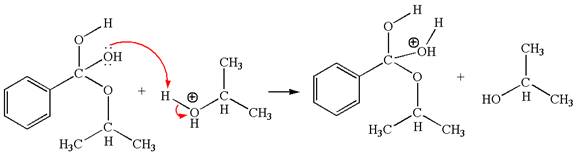
The fifth elementary step:
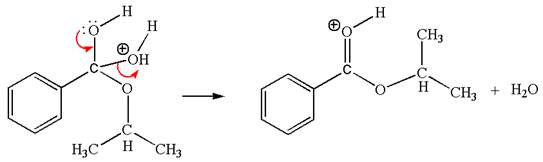
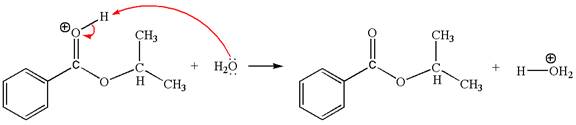
The sixth elementary step:
Explanation of Solution
The first elementary step is:
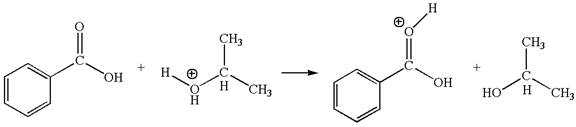
In this step, the carbonyl oxygen of benzoic acid having lone pairs is the electron-rich site. The hydrogen atom adjacent to the positively charged oxygen atom is an electron-poor site. The curved arrow mechanism for this step is shown below:
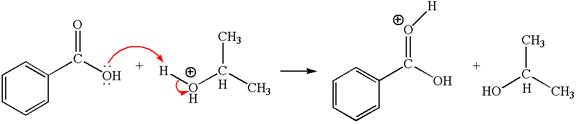
The first curved arrow is drawn from the lone pair of electron-rich oxygen to electron-poor hydrogen atom representing the formation of the
The second elementary step is:
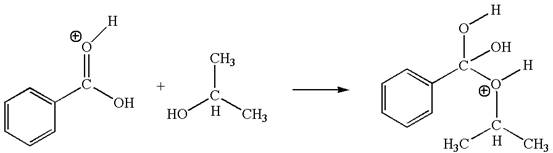
In this step, the hydroxyl oxygen of isopropanol having lone pair is the electron-rich site. The carbonyl carbon is the electron-poor site. The curved arrow mechanism for this step is shown below:
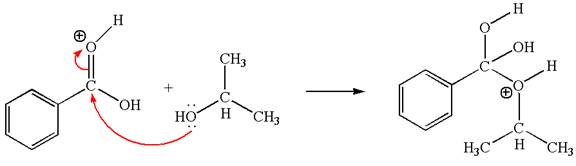
The first curved arrow is drawn from the lone pair of electron-rich hydroxyl oxygen of isopropanol to the electron-poor carbonyl carbon representing the formation of
The third elementary step is:
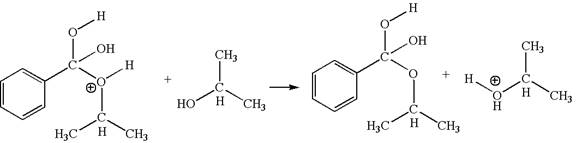 In this step, the hydroxyl oxygen of isopropannol having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The curved arrow mechanism for this step is shown below:
In this step, the hydroxyl oxygen of isopropannol having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The curved arrow mechanism for this step is shown below:

The first curved arrow is drawn from the lone pair of electron-rich hydroxyl oxygen of isopropannol to the electron-poor hydrogen atom representing the formation of
The fourth elementary step is:
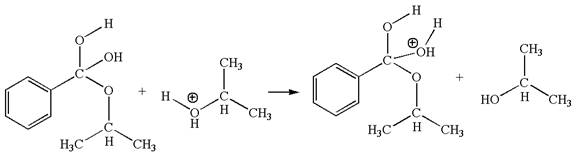 In this step, one of the hydroxyl groups bonded to the benzylic carbon is the electron-rich site. The hydrogen atom bonded to a positively charged oxygen atom is an electron-poor site. The curved arrow mechanism for this step is shown below:
In this step, one of the hydroxyl groups bonded to the benzylic carbon is the electron-rich site. The hydrogen atom bonded to a positively charged oxygen atom is an electron-poor site. The curved arrow mechanism for this step is shown below:

The first curved arrow is drawn from the lone pair of electron-rich hydroxyl oxygen atom to the electron-poor hydrogen atom indicating the formation of the
The fifth elementary step is:
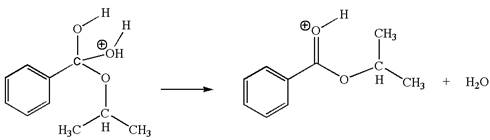
In this step, the hydroxyl oxygen having lone pairs is the electron-rich site. The carbon bonded to three oxygen atoms is the electron-poor site. The curved arrow mechanism for this step is shown below:
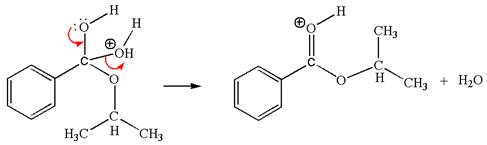
The first curved arrow is drawn from the lone pair of electron-rich hydroxyl oxygen atom to the electron-poor carbon atom indicating the formation of
The sixth elementary step is:
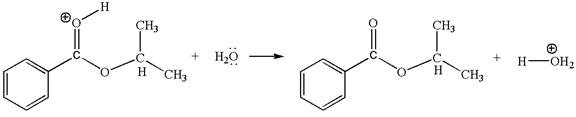
In this step, the oxygen atom of a water molecule having lone pairs is an electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The curved arrow mechanism for this step is shown below:
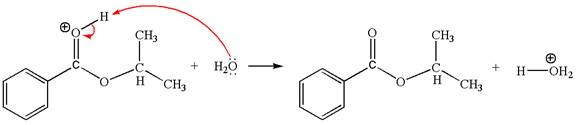
The first curved arrow is drawn from the lone pair of the electron-rich hydroxyl oxygen atom of a water molecule to the electron-poor hydrogen atom indicating the formation of the
The curved arrows for each of the given elementary steps are drawn from electron-rich site to electron-poor site and the less electronegative atom to more electronegative atom representing the formation and breaking of respective bonds.
(c)
Interpretation:
The names of each elementary step are to be identified.
Concept introduction:
In nucleophilic addition step, the nucleophile adds to the polar
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This electronegative atom forms a
An elementary step in which a proton is transferred from electron-poor site to electron-rich site and one bond is broken and another is formed simultaneously is called proton transfer step.
Answer to Problem 7.53P
The names for each of the given elementary steps are given below:
The first elementary step is proton transfer reaction.
The second elementary step is nucleophilic addition reaction.
The third elementary step is proton transfer reaction.
The fourth elementary step is proton transfer reaction.
The fifth elementary step is nucleophilic elimination reaction.
The sixth elementary step is proton transfer reaction.
Explanation of Solution
The first elementary step is:
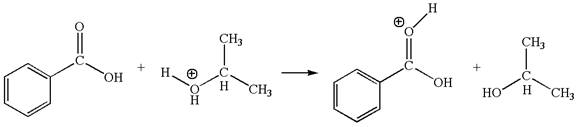
This step involved the transfer of a proton from a positively charged oxygen atom to an electron-rich carbonyl oxygen atom. Thus this step is named as proton transfer reaction.
The second elementary step is:
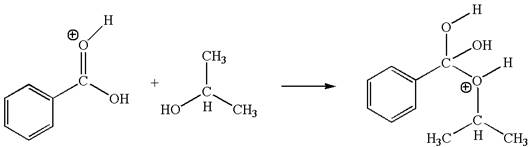
In this step, the hydroxyl oxygen of isopropyl hydrogen having lone pair is acts as nucleophile. The carbonyl carbon is electron-poor site acts as electrophile due to adjacent electronegative oxygen atom. The lone pair on nucleophilic oxygen atom approached to the electrophilic carbonyl carbon by breaking
The third elementary step is:
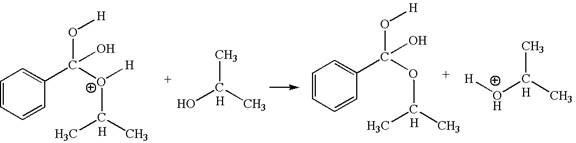 This step involved the transfer of a proton from positively charged oxygen atom to an electron-rich oxygen atom of isopropanol. Thus this step is named as proton transfer reaction.
This step involved the transfer of a proton from positively charged oxygen atom to an electron-rich oxygen atom of isopropanol. Thus this step is named as proton transfer reaction.
The fourth elementary step is:
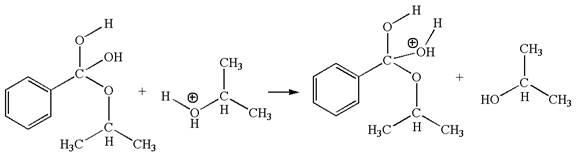
This step involved the transfer of a proton from positively oxygen atom to an electron-rich oxygen atom. Thus this step is named as proton transfer reaction.
The fifth elementary step is:
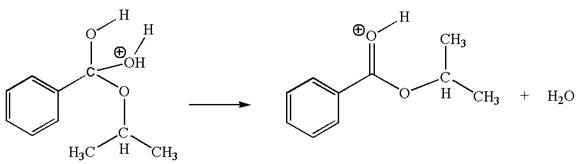
In the above step, the hydroxyl oxygen having lone pair is electron-rich site and acts as a nucleophile and the carbon atom bonded to three oxygen atoms carry partial positive charge acts as an electrophile and the water molecule is leaving group. The lone pair of nucleophilic oxygen approached to the electrophilic carbon and polar
The sixth elementary step is:
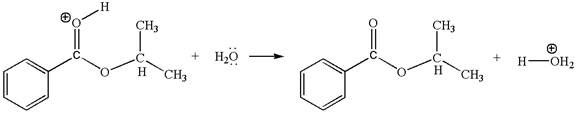
This step involved the transfer of a proton from positively carbonyl oxygen atom to the electron-rich oxygen atom of the water molecule. Thus this step is named as proton transfer reaction.
The names for the given elementary steps are identified on the basis of the type of bond-forming and breaking.
Want to see more full solutions like this?
Chapter 7 Solutions
Get Ready for Organic Chemistry
- Draw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forwardDraw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forward
- Record the IUPAC names for each of the structures shown below. a) b) c) OH d) OH e)arrow_forwardA solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at 81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the molar mass of the unknown compound. AT₁ = Km (14)arrow_forwardPlease help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forward
- Draw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.arrow_forwardComplete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forward
- Complete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forwardShow the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
