
PRINCIPLES+REACTIONS
8th Edition
ISBN: 9781337759632
Author: Masterton
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 70QAP
Complete the table on next page. 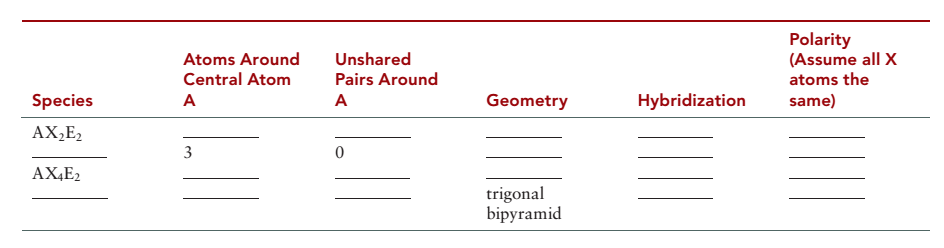
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at
equilibrium. This system is represented by the following chemical equation:
H2 (g) + Cl2 (g) → 2HCl(g)
Calculate the equilibrium constant for this reaction.
7) The pH of a 0.05M solution of HCl(aq) at 25°C is
a. 1.3
b. 2.3
c. 3.3
d. 12.7
11) The Ksp expression for copper (II) sulfate is:
a. [Cu2+][SO4²¯]
b. [Cu²+]² [SO4²]²
c. [Cu²+]²[SO4²]
d. [CuSO4]
12) Which of the following is true about a chemical system in equilibrium?
a. All chemical reactions have stopped
b. The concentration of reactants is equal to the concertation of products
c. The forward and reverse reaction rates become equal
d. The system will remain at equilibrium regardless of any external factors
Chapter 7 Solutions
PRINCIPLES+REACTIONS
Ch. 7 - Prob. 1QAPCh. 7 - Prob. 2QAPCh. 7 - Follow the directions of Question 1 for (a) IO2-...Ch. 7 - Follow the directions of Question 1 for (a) CIF4-...Ch. 7 - Follow the directions of Question 1 for (a) OCl2...Ch. 7 - Follow the directions of Question 1 for (a) C22-...Ch. 7 - Oxalic acid, H2C2O4, is a poisonous compound found...Ch. 7 - Radio astronomers have detected the isoformyl ion,...Ch. 7 - Draw Lewis structures for the following species....Ch. 7 - Follow the directions of Question 9 for the...
Ch. 7 - Dinitrogen pentoxide, N2O5, when bubbled into...Ch. 7 - Formic acid is the irritating substance that gets...Ch. 7 - Two different molecules have the formula C2H2Cl2....Ch. 7 - Two different molecules have the formula C2H6O....Ch. 7 - Prob. 15QAPCh. 7 - Prob. 16QAPCh. 7 - Write a Lewis structure for (a) XeF3+ (b) PCl4+...Ch. 7 - Write a Lewis structure for (a) BCl4 (b) ClO- (c)...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Write reasonable Lewis structures for the...Ch. 7 - Prob. 21QAPCh. 7 - Draw resonance structures for (a) SeO3 (b) CS32-...Ch. 7 - Prob. 23QAPCh. 7 - Prob. 24QAPCh. 7 - The skeleton structure for disulfur dinitride,...Ch. 7 - Borazine, B3N3H6, has the skeleton Draw the...Ch. 7 - What is the formal charge on the indicated atom in...Ch. 7 - Prob. 28QAPCh. 7 - Below are two different Lewis structures for...Ch. 7 - Below are two different Lewis structures for the...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Predict the geometry of the following species: (a)...Ch. 7 - Give all the ideal bond angles (109.5, 120, or...Ch. 7 - Prob. 38QAPCh. 7 - Prob. 39QAPCh. 7 - An objectionable component of smog is acetyl...Ch. 7 - The uracil molecule is one of the bases in DNA....Ch. 7 - Niacin is one of the B vitamins (B3). Estimate the...Ch. 7 - Which of the species with octets in Question 31...Ch. 7 - Which of the species with octets in Question 32...Ch. 7 - Which of the species with octets in Question 33...Ch. 7 - Prob. 46QAPCh. 7 - There are three compounds with the formula...Ch. 7 - There are two different molecules with the formula...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - Give the hybridization of the central atom in each...Ch. 7 - In each of the following polyatomic ions, the...Ch. 7 - Follow the directions of Question 55 for the...Ch. 7 - Give the hybridization of each atom (except H) in...Ch. 7 - Acrylonitrile, C3H3N is the building mer Orlon....Ch. 7 - What is the hybridization of nitrogen inCh. 7 - What is the hybridization of carbon inCh. 7 - Give the hybridization of the central atom...Ch. 7 - Give the hybridization of the central atom...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in the...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - Give the number of sigma and pi bonds in each...Ch. 7 - In which of the following molecules does the...Ch. 7 - Consider the pyrosulfate ion, S2O72-. It has no...Ch. 7 - Consider acetyl salicylic acid, better known as...Ch. 7 - Complete the table on next page.Ch. 7 - Given the following electro negativities...Ch. 7 - Based on the concept of formal charge, what is the...Ch. 7 - Describe the geometry of the species in which...Ch. 7 - Consider the following molecules: SiH4, PH3, H2S....Ch. 7 - Prob. 75QAPCh. 7 - In each of the following molecules, a central atom...Ch. 7 - Prob. 77QAPCh. 7 - A compound of chlorine and fluorine, CIFx, reacts...Ch. 7 - Draw the Lewis structure and describe the geometry...Ch. 7 - Consider the polyatomic ion IO65-. How many pairs...Ch. 7 - It is possible to write a simple Lewis structure...Ch. 7 - Phosphoryl chloride, POCl3, has the skeleton...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forwardPls help.arrow_forwardPls help.arrow_forward
- Pls help.arrow_forwardhelparrow_forwardDone 11:14 ⚫ worksheets.beyondlabz.com 5 (a). Using the peak information you listed in the tables for both structures, assign each peak to that portion of the structure that produces the peak in the NMR spectrum. Draw this diagram on your own sheet of paper and attach the sketch of your drawing to this question. Question 6 5 (b). Using the peak information you listed in the tables for both structures, assign each peak to that portion of the structure that produces the peak in the NMR spectrum. Draw this diagram on your own sheet of paper and attach the sketch of your drawing to this question. Question 7 6. Are there any differences between the spectra you obtained in Beyond Labz and the predicted spectra? If so, what were the differences? <arrow_forward
- 2. Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort the peaks from largest chemical shift to lowest. **Not all slots must be filled** Peak Chemical Shift (d) 5.7 1 Multiplicity multiplate .......... 5.04 double of doublet 2 4.98 double of doublet 3 4.05 doublet of quartet 4 5 LO 3.80 quartet 1.3 doublet 6 Peak Chemical Shift (d) Multiplicityarrow_forwardInterpreting NMR spectra is a skill that often requires some amount of practice, which, in turn, necessitates access to a collection of NMR spectra. Beyond Labz Organic Synthesis and Organic Qualitative Analysis have spectral libraries containing over 700 1H NMR spectra. In this assignment, you will take advantage of this by first predicting the NMR spectra for two closely related compounds and then checking your predictions by looking up the actual spectra in the spectra library. After completing this assignment, you may wish to select other compounds for additional practice. 1. Write the IUPAC names for the following two structures: Question 2 Question 3 2. Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort the peaks from largest chemical shift to lowest. **Not all slots must be filled**arrow_forward11:14 ... worksheets.beyondlabz.com 3. To check your predictions, click this link for Interpreting NMR Spectra 1. You will see a list of all the - compounds in the spectra library in alphabetical order by IUPAC name. Hovering over a name in the list will show the structure on the chalkboard. The four buttons on the top of the Spectra tab in the tray are used to select the different spectroscopic techniques for the selected compound. Make sure the NMR button has been selected. 4. Scroll through the list of names to find the names for the two compounds you have been given and click on the name to display the NMR spectrum for each. In the NMR tables below, list the chemical shift, the splitting, and the number of hydrogens associated with each peak for each compound. Compare your answers to your predictions. **Not all slots must be filled** Peak Chemical Shift (d) Multiplicity 1 2 3 4 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY