
Concept explainers
(a)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
Explanation of Solution
Given Information:
The ion is BrO-.
Here, the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule, which has nearly matching electronegativities and same number of valence electrons.
In this pattern, the bromine molecule
Here, the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
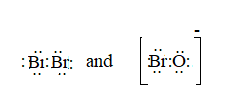
(b)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
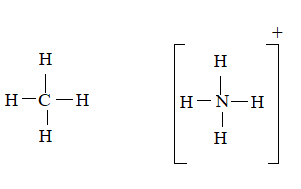
CH4 molecule
Explanation of Solution
Given Information:
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule, which has nearly matching electro negativities and same number of valance electrons.
In this pattern, the methane molecule
Here the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
(c)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
CO molecule.
Explanation of Solution
Given Information:
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule which has nearly matching electronegativities and same number of valence electrons.
In this pattern, the carbon monoxide (CO) is best suitable as:
Here, the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
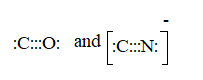
(d)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
Explanation of Solution
Given Information:
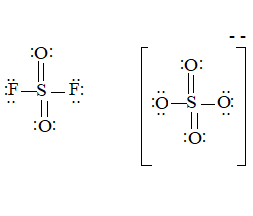
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule which has nearly matching structure.
Now, in this pattern, the anion
Here the valence electrons are:
Looking at the structure,
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
Want to see more full solutions like this?
Chapter 7 Solutions
PRINCIPLES+REACTIONS
- Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forward
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
