
Concept explainers
(a1)
To explain: The basis of the procedure for determining the number of
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a1)
Explanation of Solution
The carbon number 6 of glucose residue present in
Pictorial representation: Fig 1: represents the structure of 2,3-di-O-methylglucose.
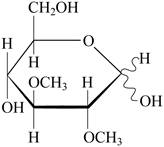
Fig 1: Structure of 2,3-di-O-methylglucose.
(a2)
To explain: What happens to the unbranched glucose residues in amylopectin during the methylation and hydrolysis procedure.
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a2)
Explanation of Solution
The polysaccharides are large
In case of unbranched residue, methylation and hydrolysis process would yield 2,3,6-tri-O-methylglucose, as the hydroxyl group at carbon 2, 3, and 6 are free.
(b)
To calculate: The percentage of glucose residues in amylopectin contained
Introduction:
The polysaccharides are large polymer molecules composed of monosaccharides unit. Starch is an example of polysaccharide that are made up of amylose and amylopectin molecule. Amylose is a linear chain of starch that are compose of glucose units. Each glucose unit in amylose are joined through α-glycosidic bond. Whereas, amylopectin is branched structure of starch that contain β-glycosidic bond.
Given: 258 mg or
(b)
Explanation of Solution
The average molecular weight of glucose is 162g/mol, then
Thus, the total weight of amylopectin in
The weight of branched residue of 2,3-di-O-methylglucose in
Thus, the average molecular weight of glucose in 2,3-di-O-methylglucose is
The percentage of glucose residue glucose residue present in amylopectin having
The weight of branched residue is
Now, substitute the value in the above given equation and calculate the percentage of glucose residue in amylopectin.
Thus, the percentage of glucose residue in amylopectin having
The percentage of glucose residue in amylopectin is 3.75 %.
Want to see more full solutions like this?
Chapter 7 Solutions
SAPLINGPLUS FOR PRINCIPLES OF BIOCHEMIS
- B- Vitamins are converted readily into important metabolic cofactors. Deficiency in any one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and is characterized by cardiac and neurological symptoms. One key diagnostic for this disease is an increased level of pyruvate and α-ketoglutarate in the bloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patient suffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardPyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardMap out all of the metabolic pathways in the liver cell. Draw out the structures and names of all compounds neatly by hand and the pathways responsible for metabolizing them. Some examples are: Glycolysis/gluconeogenesis, PPP, Glycogenesis/glycogenolysis, Krebs, ETC, selectamino acid pathways (Ala, Glu, Asp) Lipogenesis/lipolysis. Citrate/MAS/glycerol phosphate shuttlesystems, and the Cori/Glc-Ala cycles. Rules:-Draw both a mitochondrial area of metabolism and a cytoplasmic area of metabolism.-Draw the liver and its roles in glucose recycling (Cori cycle/Glc-Alanine recycling)-Avoid drawing the same molecule twice (except for separate mitochondrial/cytoplasmic populations. i.e. Design the PPP/Glycolysis so that GAP is only drawn once)-Label Carbon 4 of glucose and highlight where you would expect to find it in EVERY compound in whichit is present.-Have one or two locations for NADH/NADPH/ATP/GTP/CoQH2 – many arrows will come to/from thesespots.arrow_forward
- a. Draw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle. (Include name of Enzymes involved) b. How many rounds of Krebs will be required to waste all Carbons of Glutamic Acid as CO2? (Show by drawing out the mechanism that occurs)arrow_forwardThe malate-aspartate shuttle allows malate to be exchanged for aspartate acrossthe inner mitochondrial membrane. (a) Describe the role of the malate-aspartate shuttle in liver cells under HIGHblood glucose conditions. Be sure to explain your answer. (b) Describe the role of the malate-aspartate shuttle in liver cells under LOW blood + glucose conditions.arrow_forward(a) Write out the net reaction, calculate ∆E ̊' for the reaction, and calculate the standard free-energy change (∆G°') for the overall oxidation/reduction reaction. (h) How many moles of ATP could theoretically be generated per mole of FADH2 oxidized by this reaction, given a ∆G ̊' of ATP synthesis of + 31 kJ/mol? How many moles of ATP could be generated per mole of FADH2 oxidized by this reaction under more typical cellular conditions (where ∆G' of ATP hydrolysis is ~ -50 kJ/mol)? Be sure to show your work and explain your answer.arrow_forward
- Indicate for the reactions below which type of enzyme and cofactor(s) (if any) would be required to catalyze each reaction shown. 1) Fru-6-P + Ery-4-P <--> GAP + Sed-7-P2) Fru-6-P + Pi <--> Fru-1,6-BP + H2O3) GTP + ADP <--> GDP + ATP4) Sed-7-P + GAP <--> Rib-5-P + Xyl-5-P5) Oxaloacetate + GTP ---> PEP + GDP + CO26) DHAP + Ery-4-P <--> Sed-1,7-BP + H2O7) Pyruvate + ATP + HCO3- ---> Oxaloacetate + ADP + Piarrow_forwardThe phosphate translocase is an inner mitochondrial membrane symporter that transports H2PO4- and H+ into the mitochondrial matrix. Phosphate is a substrate for Complex V (the ATP Synthase), the enzyme that couples the synthesis of ATP to the H+ gradient formed by the electron transport chain. (a) Bongotoxin is a hypothetical compound that inhibits the phosphate translocase of the inner mitochondrial membrane. Explain why electron transport from NADH to O2 stops when bongotoxin is added to mitochondria (i.e., why do electrons stop flowing through the electron transport chain even with an abundance of NADH and O 2 present). What effect will the addition of the weak acid dinitrophenol (DNP) to the cytosol have on electron transport in bongotoxin-inhibited mitochondria? Be sure to explain your answers. (b) How much free energy is released (in kJ) when one mole of protons flows from the mitochondrial inner membrane space (IMS) to the mitochondrial matrix when the [H+ ] in the IMS is 7.9 x…arrow_forwardWhen TMPD/ascorbate is added to mitochondria as a source of electrons (TMPD/ascorbate reduce cytochrome c directly) oxygen is reduced to H2O by the electron transport chain (ETC).(a) Approximately how many ATPs would result per O2 consumed when electrons come from TMPD/ascorbate? (b) If dinitrophenol (DNP) is added to the mitochondria in (a) above, what effect would DNP have on the yield of ATPs per O2 reduced from TMPD/ascorbate electrons?arrow_forward
- Sodium fluoroacetate (FCH2CO2Na) is a very toxic molecule that is used as rodent poison. It is converted enzymatically to fluoroacetyl-CoA and is utilized by citrate synthase to generate (2R,3S)-fluorocitrate. The release of this product is a potent inhibitor of the next enzyme in the TCA cycle. Show the mechanism for the production of fluorocitrate and explain how the molecule acts as a competitive inhibitor. Predict the effect on the concentrations of TCA intermediates.arrow_forwardIn three separate experiments, pyruvate labeled with 13C at C-1, C-2, or C-3 is introduced to cells undergoing active metabolism. Trace the fate of each carbon through the TCA cycle and show when each of these carbons produces 13CO2. Glucose is similarly labeled at C-2 with 13C. During which reaction will this labeled carbon be released as 13CO2?arrow_forwardPlease draw this out and show how they react with electron flow! TPP is also utilized in transketolase reactions in the PPP. Give a mechanism for the TPP-dependent reaction between Xylulose-5-phosphate and Ribose-5-Phosphate to yield the products of Glyceraldehyde-3-phosphate and Sedoheptulose-7-Phosphate.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





