
Concept explainers
(a)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the volume is halved and the temperature and the number of particles remain same.
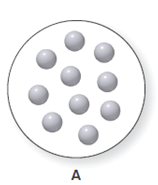
Concept Introduction:
The pressure is defined as the force which is exerted by the substance on another substance at per unit area. It is also defined as the force that is exerted by the particles of the gas on the wall of container.
(b)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the pressure is doubled and the temperature and the number of particles remain same.
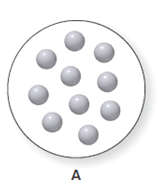
Concept Introduction:
According to Boyle's law, the change in volume results in the change in pressure at constant temperature and at constant mol of gas. It means that the pressure of sample is inversely proportional to the volume of the sample. This relation is represented as follows −
(c)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the temperature is increased and the pressure and the number of particles remain the same.
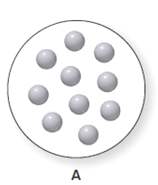
Concept Introduction:
According to Charles law, volume of gas is directly proportional to the temperature at constant pressure and at constant mol of gas which means increment in volume results in the increased value of temperature. This relation is represented as-
(d)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the number of particles is doubled and the pressure and the temperature remain same.
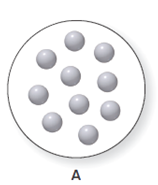
Concept Introduction:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





