
Concept explainers
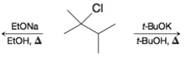
Write the structure(s) of the major product(s) obtained when 2-chloro-2,3-dimethylbutane reacts with
(a) Sodium ethoxide (EtONa) in ethanol (EtOH) ar 80°C or (in a separate reaction) with
(b) Potassium tert-butoxide (t-BuOK) in tert-butyl alcohol (t-BuOH) at 80°C.
If more than one product is formed, indicate which one would be expected to be the major product.
(c) Provide a detailed mechanism for formation of the major product from each reaction, including a drawing of the transition stare structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry (7th Edition)
Organic Chemistry (8th Edition)
Fundamentals Of Thermodynamics
Fundamentals of Physics Extended
- Draw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardDraw the mechanism for the oxidation of 3-bromo-cyclohexan-1-ol.arrow_forwardConvert the following Fischer projection to Haworth projections. show work and show the arrows please.arrow_forward
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


