
Concept explainers
(a)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
Explanation of Solution
Given Information:
The ion is BrO-.
Here, the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule, which has nearly matching electronegativities and same number of valence electrons.
In this pattern, the bromine molecule
Here, the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
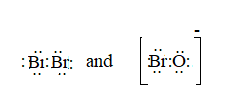
(b)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
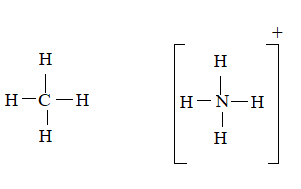
CH4 molecule
Explanation of Solution
Given Information:
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule, which has nearly matching electro negativities and same number of valance electrons.
In this pattern, the methane molecule
Here the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
(c)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
CO molecule.
Explanation of Solution
Given Information:
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule which has nearly matching electronegativities and same number of valence electrons.
In this pattern, the carbon monoxide (CO) is best suitable as:
Here, the valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
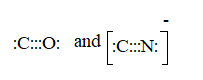
(d)
Interpretation:
The formula of the polyatomic ion needs to be determined having similar Lewis structure as the
Concept introduction:
The Lewis structures are used to write a shorthand configuration of number of available valence electrons in an atom for bonding. This structure deals with the magic number 8, and hence, the octet completion is shown by the bonded electrons between the atoms.
This structure is made on basis of octet rule understanding, and it is made sure that the number of electrons surrounding an atom must not divert from the octet.
Answer to Problem 16QAP
Explanation of Solution
Given Information:
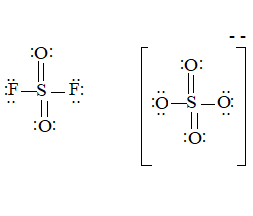
The ion is
Here the total valence electrons are:
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
A similar type of Lewis structure is possible in only such molecule which has nearly matching structure.
Now, in this pattern, the anion
Here the valence electrons are:
Looking at the structure,
Bond pair electrons are obtained as:
Similarly, lone pairs are counted as:
The Lewis structure will be drawn as:
Want to see more full solutions like this?
Chapter 7 Solutions
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
- (a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forward
- In a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
