
EBK CHEMISTRY
9th Edition
ISBN: 9780100453807
Author: ZUMDAHL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 163CP
The figure below represents part of the emission spectrum for a one-electron ion in the gas phase. All the lines result from electronic transitions from excited states to the n 3 state. (See Exercise 174.)
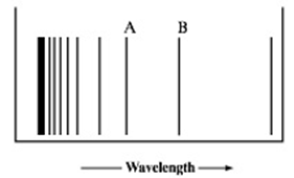
- a. What electronic transitions correspond to lines A and B?
- b. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write formulas for ionic compounds composed of the following ions.
Use units as a guide to your solutions.
24. sodium and nitrate
25. calcium and chlorate
26. aluminum and carbonate
27.
CHALLENGE Write the formula for an ionic compound formed by ions from a group 2 element and polyatomic ions composed of only carbon and oxygen.show work step by step
ADDITIONAL PRACTICE
PRACTICE Problems
Write formulas for ionic compounds composed of the following ions.
Use units as a guide to your solutions.
24. sodium and nitrate
25. calcium and chlorate
26. aluminum and carbonate
27. CHALLENGE Write the formula for an ionic compound formed by ions from a group 2 element
and polyatomic ions composed of only carbon and oxygen.
ounds 1998
7:35
<
Dji
Question 19 of 22
5G 50%
Submit
What is the pH of a buffer made from 0.350
mol of HBrO (Ka = 2.5 × 10-9) and 0.120
mol of KBRO in 2.0 L of solution?
|
1
2
3
☑
4
5
6
C
7
8 ☐ 9
+/-
Tap here for additional resources
|||
0 ×10
Г
Chapter 7 Solutions
EBK CHEMISTRY
Ch. 7 - Four types of electromagnetic radiation (EMR) are...Ch. 7 - Characterize the Bohr model of the atom. In the...Ch. 7 - What experimental evidence supports the quantum...Ch. 7 - List the most important ideas of the quantum...Ch. 7 - What are quantum numbers? What information do we...Ch. 7 - How do 2p orbitals differ from each other? How do...Ch. 7 - Four blocks of elements in a periodic table refer...Ch. 7 - What is the difference between core electrons and...Ch. 7 - Prob. 9RQCh. 7 - The radius trend and the ionization energy trend...
Ch. 7 - Prob. 1ALQCh. 7 - Defend and criticize Bohrs model. Why was it...Ch. 7 - The first four ionization energies for the...Ch. 7 - Compare the first ionization energy of helium to...Ch. 7 - Which has the larger second ionization energy,...Ch. 7 - Explain why a graph of ionization energy versus...Ch. 7 - Without referring to your text, predict the trend...Ch. 7 - Account for the fact that the line that separates...Ch. 7 - Explain electron from a quantum mechanical...Ch. 7 - Choose the best response for the following. The...Ch. 7 - Consider the following statement "The ionization...Ch. 7 - Prob. 12ALQCh. 7 - How does probability fit into the description of...Ch. 7 - What is meant by an orbital?Ch. 7 - Explain the difference between the probability...Ch. 7 - Is the following statement true or false? The...Ch. 7 - Which is higher in energy, the 2s or 2p orbital,...Ch. 7 - Prove mathematically that it is more energetically...Ch. 7 - What type of relationship (direct or inverse) e...Ch. 7 - What do we mean by the frequency of...Ch. 7 - Explain the photoelectric effectCh. 7 - Describe briefly why the study of electromagnetic...Ch. 7 - How does the wavelength of a fast-pitched baseball...Ch. 7 - The following is an energy-level diagram for...Ch. 7 - The Bohr model works for only one electron...Ch. 7 - We can represent both probability and radial...Ch. 7 - Consider the representations of the p and d atomic...Ch. 7 - The periodic table consists of four blocks of...Ch. 7 - Many times the claim is made that subshells...Ch. 7 - Prob. 30QCh. 7 - Elements with very large ionization energies also...Ch. 7 - The changes in electron affinity as one goes down...Ch. 7 - Why is it much harder to explain the line spectra...Ch. 7 - Scientists use emission spectra to confirm the...Ch. 7 - Does the minimization of electron-electron...Ch. 7 - In the hydtogen atom, what is the physical...Ch. 7 - The work function is the energy required to remove...Ch. 7 - Many more anhydrous lithium salts are hygroscopic...Ch. 7 - The laser in an audio CD player uses light with u...Ch. 7 - An FM radio station broadcasts at 99.5 MHz....Ch. 7 - Microwave radiation has a wavelength on the order...Ch. 7 - A photon of ultraviolet (UV) light possesses...Ch. 7 - Octyl methoxycinoamate and oxybenzone are common...Ch. 7 - Human color vision is " produced" by the nervous...Ch. 7 - Consider the following waves representing...Ch. 7 - One type of electromagnetic radiation has a...Ch. 7 - Carbon absorbs energy at a wavelength of 150. nm....Ch. 7 - X rays have wavelengths on the order of 1 1010 m....Ch. 7 - The work function of an element is the energy...Ch. 7 - It takes 208.4 kJ of energy to remove 1 mole of...Ch. 7 - It takes 7.21 1019 J of energy to remove an...Ch. 7 - Ionization energy is the energy required to remove...Ch. 7 - Calculate the de Broglie wavelength for each of...Ch. 7 - Neutron diffraction is used in determining the...Ch. 7 - A particle has a velocity that is 90.% of the...Ch. 7 - Calculate the velocities of electrons with de...Ch. 7 - Calculate the wavelength of light emiued when each...Ch. 7 - Calculate the wavelength of light emitted when...Ch. 7 - Using vertical lines, indicate the transitions...Ch. 7 - Using vertical lines, indicate the transitions...Ch. 7 - Calculate the longest and shortest wavelengths of...Ch. 7 - Assume that a hydrogen atoms electron has been...Ch. 7 - Does a photon of visible light ( 400 to 700 nm)...Ch. 7 - An electron is excited from the n = 1 ground state...Ch. 7 - Calculate the maximum wavelength of light capable...Ch. 7 - Consider an electron for a hydrogen atom in an...Ch. 7 - An excited hydrogen atom with an electron in the n...Ch. 7 - An excited hydrogen atom emits light with a...Ch. 7 - Using the Heisenberg uncertainty principle,...Ch. 7 - The Heisenberg uncertainty principle can be...Ch. 7 - What are the possible values for the quantum...Ch. 7 - Identify each of the following orbitals and...Ch. 7 - Which of the following sets of quantum numbers are...Ch. 7 - Which of the following sets of quantum numbers are...Ch. 7 - What is the physical significance of the value of...Ch. 7 - In defining the sizes of orbitals, why must we use...Ch. 7 - Total radial probability distributions for the...Ch. 7 - Tbe relative orbital levels for the hydrogen atom...Ch. 7 - How many orbitals in an atom can have the...Ch. 7 - How many electrons in an atom can have the...Ch. 7 - Give the maximum number of electrons in an atom...Ch. 7 - Give the maximum number of electrons in an atom...Ch. 7 - Draw atomic orbital diagrams representing the...Ch. 7 - For elements l36, there are two exceptions to the...Ch. 7 - The elements Si, Ga, As, Ge, Al, Cd, S, and Se are...Ch. 7 - The elements Cu, O, La, Y, Ba, Tl, and Bi are all...Ch. 7 - Write the expected electron configurations for...Ch. 7 - Write the expected electron configurations for...Ch. 7 - Write the expected ground-state electron...Ch. 7 - Using only the periodic table inside the front...Ch. 7 - Given the valence electron orbital level diagram...Ch. 7 - Identify the following elements. a. An excited...Ch. 7 - In the ground state of mercury, Hg, a. how many...Ch. 7 - In the ground state of element 115, Uup, a. how...Ch. 7 - Give a possible set of values of the four quantum...Ch. 7 - Give a possible set of values of the four quantum...Ch. 7 - Valence electrons are those electrons in the...Ch. 7 - How many valence electrons do each of the...Ch. 7 - A certain oxygen atom has the electron...Ch. 7 - Which of the following electron configurations...Ch. 7 - Which of elements 1-36 have two unpaired electrons...Ch. 7 - Which of elements 136 have one unpaired electron...Ch. 7 - One bit of evidence that the quantum mechanical...Ch. 7 - Identify how many unpaired electrons are present...Ch. 7 - Prob. 105ECh. 7 - Arrange the following groups of atoms in order of...Ch. 7 - Prob. 107ECh. 7 - Arrange the atoms in Exercise 108 in order of...Ch. 7 - In each of the following sets, which atom or ion...Ch. 7 - In each of the following sets, which atom or ion...Ch. 7 - Element 106 has been named seaborgium, Sg, in...Ch. 7 - The first ionization energies of As and Se are...Ch. 7 - Rank the elements Be, B, C, N, and O in order of...Ch. 7 - Consider the following ionization energies for...Ch. 7 - The following graph plots the first, second, and...Ch. 7 - For each of the following pairs of elements (C and...Ch. 7 - For each of the following pairs of elements (Mg...Ch. 7 - The electron affinities of the elements from...Ch. 7 - In the second row of the periodic table, Be, N,...Ch. 7 - Prob. 121ECh. 7 - Order the atoms in each of the following sets from...Ch. 7 - The electron affinity for sulfur is more negative...Ch. 7 - Which has the more negative electron affinity, the...Ch. 7 - Write equations corresponding to the following: a....Ch. 7 - Using data from the text, determine the following...Ch. 7 - Prob. 127ECh. 7 - Cesium was discovered in natural mineral waters in...Ch. 7 - 'The bright yellow light emitted by a sodium vapor...Ch. 7 - Does the information on alkali metals in Table 2-8...Ch. 7 - Predict the atomic number of the next alkali metal...Ch. 7 - Complete and balance the equations for the...Ch. 7 - Prob. 134ECh. 7 - "Lithium" is often prescribed as a...Ch. 7 - A carbon-oxygen double bond in a certain organic...Ch. 7 - Photogray lenses incorporate small amounts of...Ch. 7 - Mars is roughly 60 million km from the earth. How...Ch. 7 - Consider the following approximate visible light...Ch. 7 - One of the visible lines in the hydrogen emission...Ch. 7 - Using Fig. 2-30, list the elements (ignore the...Ch. 7 - Are the following statements true for the hydrogen...Ch. 7 - Although no currently known elements contain...Ch. 7 - Which of the following orbital designations are...Ch. 7 - The four most abundant elements by mass in the...Ch. 7 - Consider the eight most abundant elements in the...Ch. 7 - An ion having a 4+ charge and a mass of 49.9 u has...Ch. 7 - The successive ionization energies for an unknown...Ch. 7 - In the ground state of cadmium, Cd, a. how many...Ch. 7 - Prob. 152CWPCh. 7 - It takes 476 kJ to remove 1 mole of electrons from...Ch. 7 - Calculate, to four significant figures, the...Ch. 7 - Assume that a hydrogen atoms electron bas been...Ch. 7 - Determine the maximum number of electrons that can...Ch. 7 - Consider the ground state of arsenic, As. How many...Ch. 7 - Which of the following statements is(are) true? a....Ch. 7 - Identify the following three elements. a. The...Ch. 7 - For each of the following pairs of elements,...Ch. 7 - Which of the following statements is(are) true? a....Ch. 7 - Three elements have the electron configurations...Ch. 7 - The figure below represents part of the emission...Ch. 7 - One of the emission spectral lines for Be3+ has a...Ch. 7 - The figure below represents part of the emission...Ch. 7 - When lhe excited electron in a hydrogen atom falls...Ch. 7 - Prob. 167CPCh. 7 - For hydrogen atoms, the wave function for the...Ch. 7 - The wave function for the 2pz, orbital in the...Ch. 7 - Answer the following questions, assuming that ms,...Ch. 7 - Assume that we are in another universe with...Ch. 7 - Without looking at data in the text, sketch a...Ch. 7 - The following numbers are the ratios of second...Ch. 7 - We expect the atomic radius to increase going down...Ch. 7 - The ionization energy for a 1s electron in a...Ch. 7 - An atom of a particular element is traveling at...Ch. 7 - As the weapons officer aboard the Srarship...Ch. 7 - Answer the following questions based on the given...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- aw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. B C Br HO O Substitution will not occur at a significant rate. Explanation Check + Х Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibarrow_forwardComplete the following reactions with the necessary reagents to complete the shown transformation. Example: 1. 2. ? 3. 018 Br OH Answer: H₂O, H2SO4, HgSO4arrow_forward7:34 • < Question 18 of 22 5G 50% Submit What is the pH of a buffer made from 0.220 mol of HCNO (Ka = 3.5 × 10-4) and 0.410 mol of NaCNO in 2.0 L of solution? 1 2 3 ☑ 4 5 6 C 7 8 | 9 +/- 0 ×10 Tap here for additional resources ||| Гarrow_forward
- 6:46 ✔ 5G 58% < Question 7 of 22 Submit What is the primary species in solution at the halfway point in a titration of NH3 with HBr? A NH3 and H+ B NH₁+ and H+ C NH4+ D NH3 and NH4+ Tap here for additional resources |||arrow_forward6:49 Dji < Question 15 of 22 4G 57% Submit The pOH of a solution is 10.50. What is the OH- concentration in the solution? A 3.2 × 10-4 M B C 3.2 x 10-11 M 10.50 M D 4.2 M E 3.50 M Tap here for additional resources |||arrow_forwardヨ 6:49 Dji < Question 13 of 22 5G 57% Submit The pH of a solution is 2.40. What is the H+ concentration in the solution? A B 2.5 x 10-12 M 4.0 × 10-3 M C 2.40 M D 4.76 M 11.60 M Tap here for additional resources |||arrow_forward
- ヨ C 6:48 Di✔ < Question 12 of 22 5G 57% Submit The pH of a solution is 12.50. What is the H+ concentration in the solution? A 0.032 M B 3.2 × 10-13 M 1.5 M D 9.25 M 12.50 M Tap here for additional resources |||arrow_forwardヨ C 6:48 Di✔ < Question 11 of 22 5G 57% Submit The pH of a solution is 1.50. What is the H+ concentration in the solution? A 0.032 M B 3.2 × 10-13 M 1.5 M D 2.15 M 12.50 M Tap here for additional resources |||arrow_forwardSelect the product of the following reaction. Lon HO Meat ?? CH₂OH OH A D OH OCH B OH of OCH of CH חח E C CHarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardUse excel to plot the following titration data. Once you have done your plot, make sure to label the axes correctly. Use your graph to determine the pK, for the weak acid. Attach your plot to the back of this worksheet. A 1.0M solution of weak acid was titrated with a base and the following data was collected. Equivalents of Base pH observed 0.05 3.4 0.15 3.9 0.25 4.2 0.40 4.5 0.60 4.9 0.75 5.2 0.85 5.4 0.95 6.0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY