
Concept explainers
(a)
Interpretation:
An oxygen-binding curve for a hypothetical two subunit hemoglobin with
Concept introduction:
Hill equation is represented as follows:
Here, Y- fractional saturation
n − a measure of the degree of cooperativity in ligand binding.
P50 − partial pressure of oxygen at which hemoglobin is half saturated.
The Hill plot of log(Y/1-Y) versus log(P50) should be a linear graph with slope of n.
Answer to Problem 14P
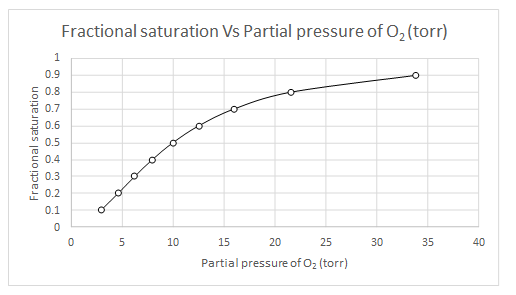
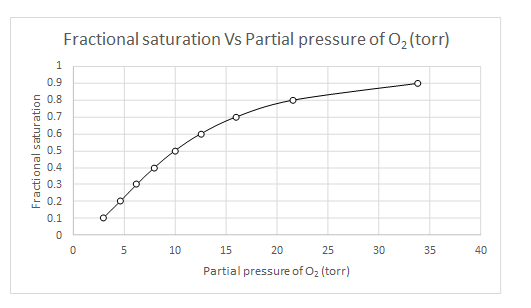
Explanation of Solution
The values of
| Y | log(Y/(1-Y) | log(Y/(1-Y)-n log (P50) | log (pO2) | pO2 |
| 0.1 | -0.95424251 | 0.845757491 | 0.469865 | 2.950294 |
| 0.2 | -0.60205999 | 1.197940009 | 0.665522 | 4.629374 |
| 0.3 | -0.36797679 | 1.432023215 | 0.795568 | 6.245518 |
| 0.4 | -0.17609126 | 1.623908741 | 0.902172 | 7.983099 |
| 0.5 | 0 | 1.8 | 1 | 10 |
| 0.6 | 0.176091259 | 1.976091259 | 1.097828 | 12.52646 |
| 0.7 | 0.367976785 | 2.167976785 | 1.204432 | 16.01148 |
| 0.8 | 0.602059991 | 2.402059991 | 1.334478 | 21.60119 |
| 0.9 | 0.954242509 | 2.754242509 | 1.530135 | 33.89493 |
(b)
Interpretation:
An oxygen-binding curve for shypothetical two subunit hemoglobin with
Concept introduction:
Concerted model equation is written as follows:
Here, Y − fractional saturation
a − ratio between the substrate concentration and the dissociation constant for a ligand binding to a single site in R state.
L − The ratio of the concentrations of the T and R states with no ligands bound
c − the ratio between the dissociation constant for a ligand binding to a single site in R state and that of T state.
n − number of binding sites
Also, the ratio can be calculated as follows:
Here,
pO2 − partial pressure of oxygen
KR - the dissociation constant for a ligand binding to a single site in R state.
Answer to Problem 14P
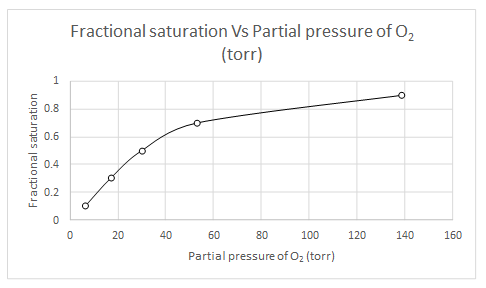
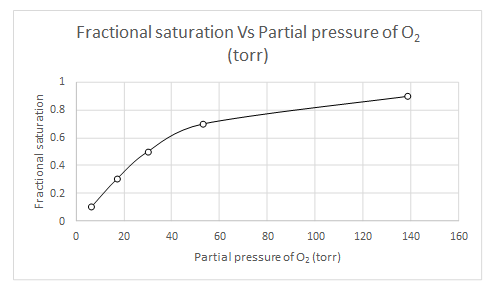
Explanation of Solution
When
Likewise, partial pressure of oxygen for several fractional saturation values are calculated and a plot of fractional saturation versus oxygen partial pressure is drawn.
| Y | pO2 |
| 0.1 | 6.55 |
| 0.3 | 17.09 |
| 0.5 | 30.15 |
| 0.7 | 53.21 |
| 0.9 | 138.91 |
Want to see more full solutions like this?
- What is the formation of glycosylated hemoglobin (the basis for the HbA1c test)? Can you describe it?arrow_forwardPlease analze the gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Also use a ruler to measure the length of the column in centimeters and calculate the MW of each band observed. Lane 1: buffer Lane 2 : Ladder Lane 3: Lysate Lane 4: Flowthrough Lane 5: Wash Lanes 6-8: E1, E2, E3 Lane 9: Dialyzed VRK1 Lane 10: LDHarrow_forwardDo sensory neurons express ACE2 or only neurolipin-1 receptors for COVID19 virus particle binding?arrow_forward
- Explain the process of CNS infiltration of COVID19 through sensory neurons from beginning to end, including processes like endocytosis, the different receptors/proteins that are involved, how they are transported and released, etc.,arrow_forwardH2C CH2 HC-COOO CH2 ܘHO-C-13c-O isocitrate C-S-COA H213c CH2 C-OO 13C-S-COA CH2 C-00 the label will not be present in succinyl CoA C-S-COA succinyl-CoAarrow_forwardA culture of kidneys cells contains all intermediates of the citric acid cycle. It is treated with an irreversible inhibitor of malate dehydrogenase, and then infused withglucose. Fill in the following list to account for the number of energy molecules that are formed from that one molecule of glucose in this situation. (NTP = nucleotidetriphosphate, e.g., ATP or GTP)Net number of NTP:Net number of NADH:Net number of FADH2:arrow_forward
- 16. Which one of the compounds below is the final product of the reaction sequence shown here? OH A B NaOH Zn/Hg aldol condensation heat aq. HCI acetone C 0 D Earrow_forward2. Which one of the following alkenes undergoes the least exothermic hydrogenation upon treatment with H₂/Pd? A B C D Earrow_forward6. What is the IUPAC name of the following compound? A) (Z)-3,5,6-trimethyl-3,5-heptadiene B) (E)-2,3,5-trimethyl-1,4-heptadiene C) (E)-5-ethyl-2,3-dimethyl-1,5-hexadiene D) (Z)-5-ethyl-2,3-dimethyl-1,5-hexadiene E) (Z)-2,3,5-trimethyl-1,4-heptadienearrow_forward
- Consider the reaction shown. CH2OH Ex. CH2 -OH CH2- Dihydroxyacetone phosphate glyceraldehyde 3-phosphate The standard free-energy change (AG) for this reaction is 7.53 kJ mol-¹. Calculate the free-energy change (AG) for this reaction at 298 K when [dihydroxyacetone phosphate] = 0.100 M and [glyceraldehyde 3-phosphate] = 0.00300 M. AG= kJ mol-1arrow_forwardIf the pH of gastric juice is 1.6, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the membrane potential across this membrane is -70.0 mV and the temperature is 37 °C. AG= kJ mol-1arrow_forwardConsider the fatty acid structure shown. Which of the designations are accurate for this fatty acid? 17:2 (48.11) 18:2(A9.12) cis, cis-A8, A¹¹-octadecadienoate w-6 fatty acid 18:2(A6,9)arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





