
Concept explainers
a)
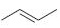
Interpretation:
The reaction of the alkene, 2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
b)
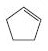
Interpretation:
The reaction of the alkene, cyclopentene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, cyclopentene, with a proton to give a carbocation and also to give the structure of the carbocation.
c)
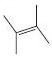
Interpretation:
The reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry - With Access (Custom)
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
