
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.SE, Problem 35AP
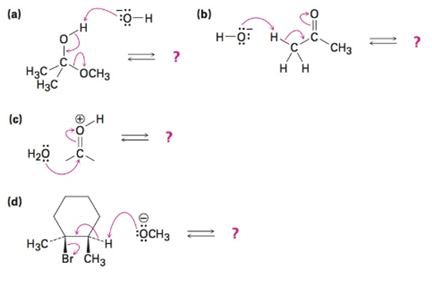
Follow the flow of electrons indicated by the curved arrows in each of the following polar reactions, and predict the products that result:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the most likely mechanism for the following
d.
'Ph
Draw the most likely mechanism for the following
Chapter 6 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 6.1 - Prob. 1PCh. 6.3 - Prob. 2PCh. 6.3 - Using curved fishhook arrows, propose a mechanism...Ch. 6.4 - Prob. 4PCh. 6.4 - An electrostatic potential map of boron...Ch. 6.5 - What product would you expect from reaction of...Ch. 6.5 - Reaction of HBr with 2-methylpropene yields...Ch. 6.6 - Prob. 8PCh. 6.6 - Predict the products of the following polar...Ch. 6.7 - Which reaction is more energetically favored, one...
Ch. 6.7 - Prob. 11PCh. 6.9 - Which reaction is faster, one with ∆G‡ = +45...Ch. 6.10 - Prob. 13PCh. 6.SE - Prob. 14VCCh. 6.SE - Prob. 15VCCh. 6.SE - Prob. 16VCCh. 6.SE - Look at the following energy diagram: (a) Is...Ch. 6.SE - Look at the following energy diagram for an...Ch. 6.SE - What is the difference between a transition state...Ch. 6.SE - Prob. 20EDRMCh. 6.SE - Prob. 21EDRMCh. 6.SE - Draw an energy diagram for a two-step exergonic...Ch. 6.SE - Draw an energy diagram for a reaction with keq =...Ch. 6.SE - The addition of water to ethylene to yield ethanol...Ch. 6.SE - When isopropylidenecyclohexane is treated with...Ch. 6.SE - Prob. 26EDRMCh. 6.SE - Draw the electron-pushing mechanism for each...Ch. 6.SE - Draw the complete mechanism for each polar...Ch. 6.SE - Prob. 29EDRMCh. 6.SE - Identify the functional groups in the following...Ch. 6.SE - Identify the following reactions as additions,...Ch. 6.SE - Identify the likely electrophilic and nucleophilic...Ch. 6.SE - For each reaction below identify the electrophile...Ch. 6.SE - Prob. 34APCh. 6.SE - Follow the flow of electrons indicated by the...Ch. 6.SE - Prob. 36APCh. 6.SE - Prob. 37APCh. 6.SE - Despite the limitations of radical chlorination of...Ch. 6.SE - Prob. 39APCh. 6.SE - Answer question 6-39 taking all stereoisomers into...Ch. 6.SE - Prob. 41APCh. 6.SE - Prob. 42APCh. 6.SE - Prob. 43APCh. 6.SE - The reaction of hydroxide ion with chloromethane...Ch. 6.SE - Prob. 45APCh. 6.SE - Ammonia reacts with acetyl chloride (CH3COCl) to...Ch. 6.SE - The naturally occurring molecule α-terpineol is...Ch. 6.SE - Prob. 48APCh. 6.SE - Prob. 49APCh. 6.SE - Draw the structures of the two carbocation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. OH H₂N-O -Ph H+ acyclic productarrow_forwardeks.com/aleksogi/x/sl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvTCeeBZbufuBYTI0Hz7m7D3ZS17Hd6m-HIl6n52njJN-TXdQA2X9yID-1SWQJTgnjARg30 111 States of Matter Understanding conceptual components of the enthalpy of solution 0/5 Ge A small amount of acetonitrile (CH, CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketched below. (These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.) CH,CN H₂O B 88 C Use these sketches to answer the questions in the table below. The enthalpy of solution AH is negative soln when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. Would heat be absorbed or released if the system moved from Stage C to D? What force would oppose or favor the system moving from Stage C to D? Check all that apply. 1 absorbed O released neither absorbed nor released. none O ionic bonding force covalent bonding force…arrow_forwardIn a system with an anodic overpotential, the variation of ŋ as a function of the current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Find the range of current densities for which the overpotential has the same value as when calculated for cases 1 and 2 (maximum relative difference of 5% with respect to the behavior for higher fields). To which overpotential range does this correspond? Data: 10 = 1.5 mA cm², T = 300°C, ẞ = 0.64, R = 8.314 J K 1 mol¹ and F = 96485 C mol-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License