
Concept explainers
(a)
Interpretation: The arrow pushing method is to be interpreted for the given transformation:
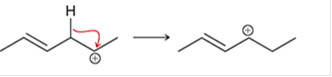
Concept introduction: Intermediates like the carbocation can rearrange to give more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary This transformation can occur with the hydride shift; methylene shifts or methyl shift. The arrow pushing method helps to identify the curved arrows and transformation.
(b)
Interpretation: The arrow pushing method is to be interpreted for the given transformation.
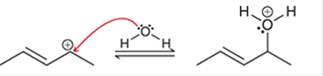
Concept introduction: Intermediates like carbocation can rearrange to give more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary This transformation can occur with the hydride shift; methylene shifts or methyl shift. The arrow pushing method helps to identify the curved arrows and transformation.
(c)
Interpretation: The arrow pushing method is to be interpreted for the given transformation.
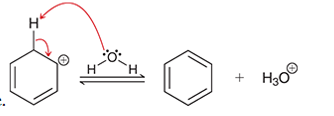
Concept introduction: Intermediates like carbocation can rearrange to give more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary This transformation can occur with the hydride shift; methylene shifts or methyl shift. The arrow pushing method helps to identify the curved arrows and transformation.
(d)
Interpretation: The arrow pushing method is to be interpreted for the given transformation.
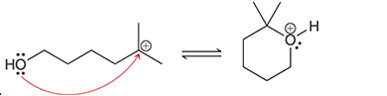
Concept introduction: Intermediates like carbocation can rearrange to give more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary This transformation can occur with the hydride shift; methylene shifts or methyl shift. The arrow pushing method helps to identify the curved arrows and transformation.
(e)
Interpretation: The arrow pushing method is to be interpreted for the given transformation.
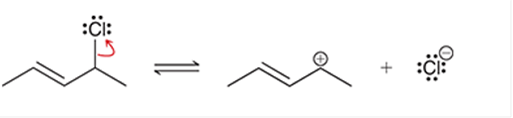
Concept introduction: Intermediates like carbocation can rearrange to give more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary This transformation can occur with the hydride shift; methylene shifts or methyl shift. The arrow pushing method helps to identify the curved arrows and transformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY (LL)-W/WILEYPLUS
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



