
Concept explainers
Interpretation:
The most stable chair conformation for the given compound has to be drawn.
Concept Introduction:
When a compound contains a cyclohexane ring in it, then there are three possibilities of conformations. They are chair, boat and twist-boat. Among these three conformations of cyclohexane ring, the most stable one is the chair conformation.

In the above structure, there are twelve substituents that can be placed. Among the twelve, six are axial substituents and six are equatorial substituents. The axial substituents can be represented as shown below,
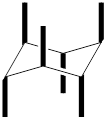
The equatorial substituents can be represented as,
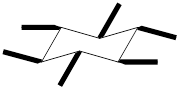
To draw a group in a correct configuration, we have to understand what is coming towards us and what is going away from us (wedge and dash bonds).
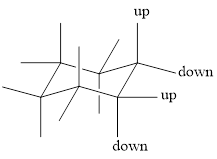
For drawing the other chair conformation, a ring flip has to be done. Ring flipping makes the skeleton as shown below,

During this ring flip from one chair conformation to another, all the substituents present in axial position goes to equatorial position and vice-versa.
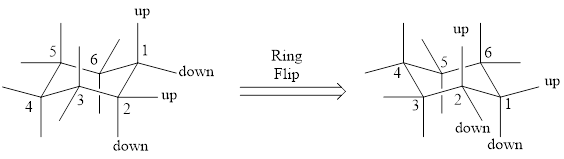
The most stable conformation among the two chair conformations can be found by looking into the bulky groups that are present in the ring. The chair conformation which has the bulky group in equatorial position will be the more stable one as there wont be steric interaction. If the bulky group is present in axial position, then there will be axial-axial interaction, which makes the conformation less stable. In simple words, we can say that the bulky group will prefer to be in equatorial position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- 7. Use Pauling's electronegativity values (Table 1.7) and Ketelaar triangle (Fig. 2.28) to classify bonding in: (3 points) a) CIF3 b) ZnCl2 c) PbSarrow_forward7. What is the IUPAC name of the following compound? A) (R)-1-oxo-2-butanol C) (R)-2-hydroxybutanal E) (S)-1-formyl-1-propanol B) (S)-1-oxo-2-butanol D) (S)-2-hydroxybutanal OH Harrow_forwardCual es la formula semidesarrollada del 3-metil-1-butino?arrow_forward
- 2. A graph shown below shows first ionization energies for elements from H to Ne. First ionization energy/kJ mol 2500 2000 1500 1000 500 T T T T 1 2 3 5 6 7 8 9 10 Atomic number a) Using arguments of electronic structure, explain why ionization energy of Li is much lower than that of H. (2 points) then dips at O. b) Using the same arguments, explain why ionization energy increases from B to N, and (3 points)arrow_forwardGive the name of this compound, including stereochemistry if relevant: CICH2 CH3 Br CH₂CH=CH2 Write in the product, including stereochemistry where relevant, for these reactions. See end of ch. 8, p. 301-303. 1. 03 a) 2-methyl-2-pentene -> 2. Zn, H* Br2 b) 1-ethylcyclopentene -->arrow_forwardNonearrow_forward
- 3. You may want to read paragraph 1.5 in your textbook before answering this question. Give electron configuration (short-hand notation is fine) for: (5 points) 3+ a) Manganese atom and Mn³+ b) Se atom c) Cu atom and Cu+arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forward
- Nonearrow_forwardHowever, why are intermolecular forces in metallic and ionic compounds not discussed as extensively? Additionally, what specific types of intermolecular attractions exist in metals and ionic compoundsarrow_forwardWhat is the preparation of 1 Liter of 0.1M NH4Cl buffer at pH 9.0 with solid NH4Cl and 0.1M NaOH. How would I calculate the math to describe this preparation? How would I use Henderson-Hasselbach equation?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





