
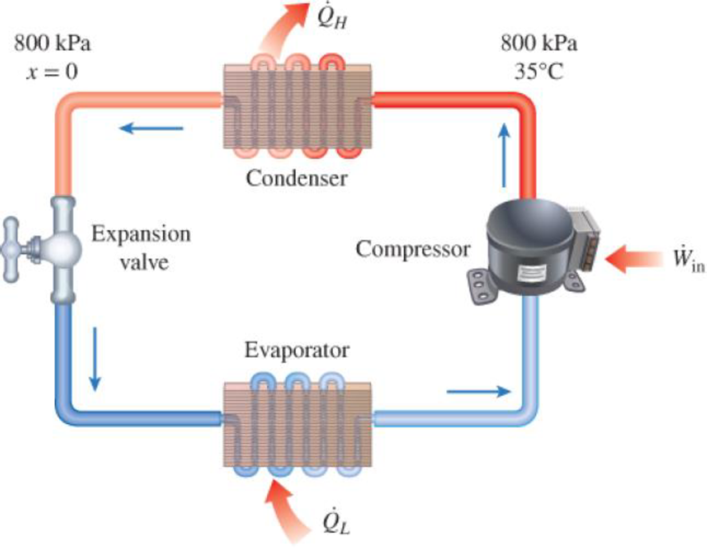
Refrigerant-134a enters the condenser of a residential heat pump at 800 kPa and 35°C at a rate of 0.018 kg/s and leaves at 800 kPa as a saturated liquid. If the compressor consumes 1.2 kW of power, determine (a) the COP of the heat pump and (b) the rate of heat absorption from the outside air.
FIGURE P6–57
(a)
The COP of the heat pump.
Answer to Problem 57P
The COP of the heat pump is
Explanation of Solution
Write the expression for the energy balance equation.
Here, the total energy entering the system is
Simplify Equation (II) and write energy balance relation of refrigrent-134a.
Here, the rate of work to be done into the system is
Substitute
Write the expression for the rate of coefficient performance of a heat pump.
Here the rate of required input of the heat pump is
Conclusion:
Convert the unit of pressure from kPa to MPa.
Refer to Table A-13, “Superheated refrigerant-134a”, obtain the below properties at the superheated pressure and temperature of 800 kPa (0.80 MPa) and 35 C using interpolation method of two variables.
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y are superheated temperature and specific enthalpy.
Show the temperature at 31.31 C and 40 C as in Table (1).
| Temperature, C | Specific enthaply, |
| Saturated liquid, | |
| 31.31 C | 267.34 |
| 35 C | ? |
| 40 C | 276.46 |
Calculate superheated pressure and temperature of 800 kPa (0.80 MPa) and 35 C for liquid phase using interpolation method.
Substitute 31.31 C for
From above calculation the initial enthalpy of condenser is
Refer to Table A-12, “Saturated pressure table” obtain properties at the superheated pressure and quality of final state of 800 kPa and 0.
Write the expression of final specific enthalpy of a two-phase system for condenser.
Here, the specific enthalpy of condenser is
Substitute
Substitute
Substitute
Thus, the COP of the heat pump is
(b)
The rate of heat absorbed from the outside air.
Answer to Problem 57P
The rate of heat absorbed from the outside air is
Explanation of Solution
Write the expression for the rate of conversation of energy principle for refrigerant 134a.
Here, the rate of heat rejected in the condenser is
Conclusion:
Substitute
Thus, the rate of heat absorbed from the outside air is
Want to see more full solutions like this?
Chapter 6 Solutions
THERMODYNAMICS-SI ED. EBOOK >I<
- my ID# 016948724. Please solve this problem step by steparrow_forwardMy ID# 016948724 please find the forces for Fx=0: fy=0: fz=0: please help me to solve this problem step by steparrow_forwardMy ID# 016948724 please solve the proble step by step find the forces fx=o: fy=0; fz=0; and find shear moment and the bending moment diagran please draw the diagram for the shear and bending momentarrow_forward
- My ID#016948724 please solve this problems and show me every step clear to follow pleasearrow_forwardMy ID# 016948724arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward[Q2]: The cost information supplied by the cost accountant is as follows:Sales 20,00 units, $ 10 per unitCalculate the (a/ newsale guantity and (b) new selling price to earn the sameVariable cost $ 6 per unit, Fixed Cost $ 30,000, Profit $ 50,000profit ifi) Variable cost increases by $ 2 per unitil) Fixed cost increase by $ 10,000Ili) Variable cost increase by $ 1 per unit and fixed cost reduces by $ 10,000arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

