
(a)
Interpretation:
The following data were collected for the given reaction at
Concept Introduction:
Rate of a reaction: It represents the speed at which a
Rate of reaction depends on time, temperature, pressure, concentration, and
Rate of the reaction is the change in the concentration of reactant or a product with time. It can be varied in accordance with temperature, pressure, concentration, presence of catalyst, surface area.
General rate reaction is,
The negative sign indicates the reduction of concentration of reactant.
Integrated rate law for first order reaction:
Consider A as substance, that gives the product based on the equation,
Where a= stoichiometric co-efficient of reactant A.
Consider the reaction has first-order rate law,
The integrated rate law equation can be given as,
The above expression is called integrated rate law for first order reaction.
(a)
Explanation of Solution
Given reaction is,
Record the given data’s
Let us consider the first order reaction
Rate constant is,
Given values are plugging above equation,
Hence, the all rate constant are nearly same, so this reaction confirmed to be first order reaction.
(b)
Interpretation:
Plot the given data in an appropriate fashion, the rate constant has to be determined.
Concept Introduction:
Rate of a reaction: It represents the speed at which a chemical reaction runs. How much concentration of substrates (reactants) consumed and how much concentration of targets (products) formed in a unit of time is said to be rate of reaction.
Rate of reaction depends on time, temperature, pressure, concentration, and
Rate of the reaction is the change in the concentration of reactant or a product with time. It can be varied in accordance with temperature, pressure, concentration, presence of catalyst, surface area.
General rate reaction is,
The negative sign indicates the reduction of concentration of reactant.
(b)
Explanation of Solution
Given reaction is,
Record the given data’s
In order to determine the rate law, we need to determine the order of the reaction with respect to
For a zeroth order reaction
In the present case a plot
The rate law is thus
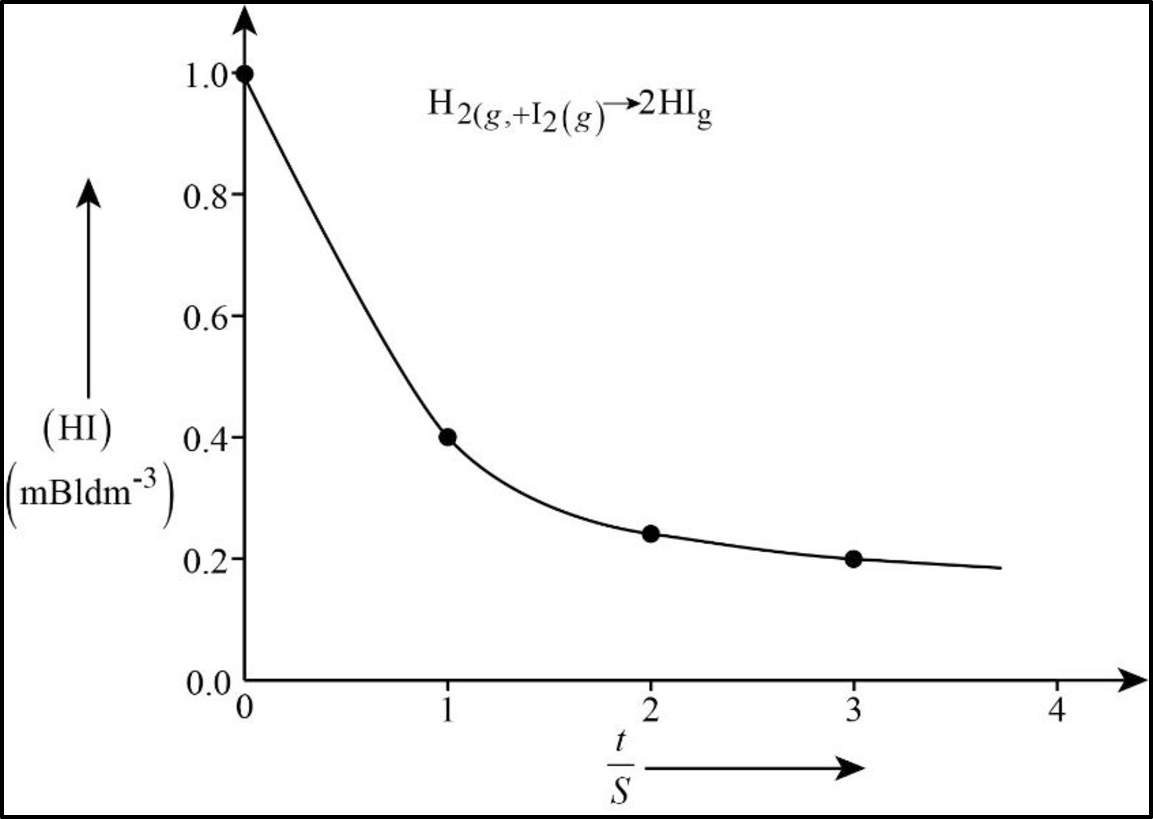
Figure 1
Want to see more full solutions like this?
Chapter 6 Solutions
Elements of Physical Chemistry
- Give the expected major product of reaction of 2,2-dimethylcyclopropane with each of the following reagents. 2. Reaction with dilute H₂SO, in methanol. Select Draw Templates More CHC Erase QQQ c. Reaction with dilute aqueous HBr. Select Drew Templates More Era c QQQ b. Reaction with NaOCH, in methanol. Select Draw Templates More d. Reaction with concentrated HBr. Select Draw Templates More En a QQQ e. Reaction with CH, Mg1, then H*, H₂O 1. Reaction with CH,Li, then H', H₂Oarrow_forwardWrite the systematic name of each organic molecule: structure O OH OH name X ☐arrow_forwardMacmillan Learning One of the molecules shown can be made using the Williamson ether synthesis. Identify the ether and draw the starting materials. А со C Strategy: Review the reagents, mechanism and steps of the Williamson ether synthesis. Determine which of the molecules can be made using the steps. Then analyze the two possible disconnection strategies and deduce the starting materials. Identify the superior route. Step 6: Put it all together. Complete the two-step synthesis by selecting the reagents and starting materials. C 1. 2. Answer Bank NaH NaOH NaOCH, снен, сен, он Сиси, Сне (СН), СОН (Сн, Свarrow_forward
- Write the systematic name of each organic molecule: structure CH3 O CH3-CH-CH-C-CH3 OH HV. CH3-C-CH-CH2-CH3 OH CH3 O HO—CH, CH–CH—C CH3 OH 오-오 name X G ☐arrow_forwardHI Organic Functional Groups Predicting the reactants or products of esterification What is the missing reactant in this organic reaction? HO OH H +回 + H₂O 60013 Naomi V Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click and drag to start drawing a structure. Explanation Check 1 2 #3 $ 4 2025 % ala5 'a :☐ G & 67 8 Ar K enter Accessible 9 Q W E R TY U 1 tab , S H J Karrow_forwardPlease help me with number 5 using my data and graph. I think I might have number 3 and 4 but if possible please check me. Thanks in advance!arrow_forward
- dict the major products of this organic reaction. C Explanation Check 90 + 1.0₂ 3 2. (CH3)2S Click and drag f drawing a stru © 2025 McGraw Hill LLC. All Rights Reserved. • 22 4 5 7 8 Y W E R S F H Bilarrow_forwardcan someone draw out the reaction mechanism for this reaction showing all the curly arrows and 2. Draw the GPNA molecule and identify the phenylalanine portion. 3. Draw L-phenylalanine with the correct stereochemistryarrow_forwardWhat is the reaction mechanism for this?arrow_forward
- Predict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. esc esc Explanation Check 2 : + + X H₁₂O + Х ง WW E R Y qab Ccaps lock shift $ P X Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil T FR F18 9 G t K L Z X V B N M control opption command command T C darrow_forwardDraw the Markovnikov product of the hydrohalogenation of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for caps lock Explanation Check 2 W E R + X 5 HCI Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil Y F G H K L ZZ X C V B N M control opption command F10 F10 command 4 BA Ar Carrow_forwardI don't understand why the amide on the top left, with the R attached to one side, doesn't get substituted with OH to form a carboxylic acid. And if only one can be substituted, why did it choose the amide it chose rather than the other amide?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





