
Concept explainers
Interpretation: The resonance structure of diazomethane should be found.
Concept Introduction: Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance.
In some molecules, there is possibility of more than one Lewis structure where all the structures are equally acceptable. One of the acceptable Lewis structures of these molecules is called resonance structure.
All the possible resonance structures are imaginary whereas the resonance hybrid is real.
Any of the possible structure does not exist as such like a stable real molecule. So it is not possible to isolate one resonance structure.
These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Structure with greater number of covalent bonds are more stable comparing to that with lower number of covalent bonds.
Structure which does not involve charge separation is more stable when comparing with structure having positive and negative charge separation.
While drawing resonance structure of a molecule some rules should be followed where the position, over whole charge and chemical framework remains intact. Also only π and nonbonding electron has been moved in all the three resonance structures
Answer to Problem 6.36QP
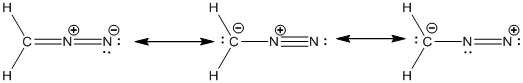
Explanation of Solution
The given atomic arrangement of diazomethane is
Resonance structure of hydrazoic acid is drawn below.

In the case of diazomethane, the chemical bonding of a molecule cannot be represented using a single Lewis structure. The chemical bonding are described by delocalization of electrons forming 3 possible resonance structures. In all the 3 resonance structures the position, over whole charge and chemical framework remains intact.
The resonance structures of the diazomethane molecule were drawn.
Interpretation: The formal charge of diazomethane molecule should be found.
Concept Introduction:
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.36QP
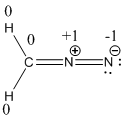
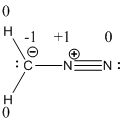
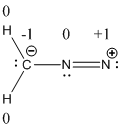
Resonance structure of diazomethane is given below.

Explanation of Solution
The formal charge of the given compound is calculated,
- First hydrogen atom
Substituting these values to the equation,
- Second hydrogen atom
Substituting these values to the equation,
- Carbon atom
Substituting these values to the equation,
- First nitrogen atom,
Substituting these values to the equation,
- Second nitrogen atom
Substituting these values to the equation,
Resonance structure of diazomethane is given below.
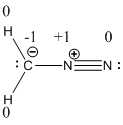
The formal charge of the given compound is calculated,
- First hydrogen atom
Substituting these values to the equation,
- Second hydrogen atom
Substituting these values to the equation,
- Carbon atom
Substituting these values to the equation,
- First nitrogen atom,
Substituting these values to the equation,
- Second nitrogen atom
Substituting these values to the equation,
Resonance structure of the diazomethane is given below.

The formal charge of the given compound is calculated,
- First hydrogen atom
Substituting these values to the equation,
- Second hydrogen atom
Substituting these values to the equation,
- Carbon atom
Substituting these values to the equation,
- First nitrogen atom,
Substituting these values to the equation,
- Second nitrogen atom
Substituting these values to the equation,
The formal charges of diazomethane molecule were shown.
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: Atoms First
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





