
ORGANIC CHEMISTRY
9th Edition
ISBN: 9780134645704
Author: WADE AND SIMEK
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.34SP
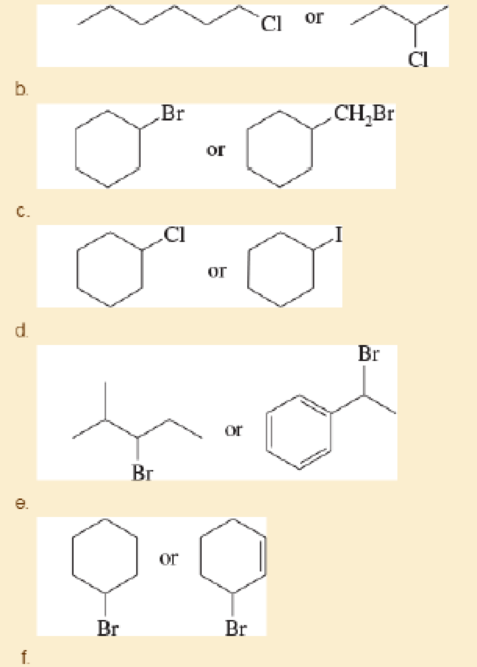
Predict the compound in each pair that will undergo solvolysis (in aqueous ethanol) more rapidly a (CH3CH2)2CH—Cl or (CH3)3C—Cl
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 6 Solutions
ORGANIC CHEMISTRY
Ch. 6.1 - Classify each compound as an alkyl halide, a vinyl...Ch. 6.2 - Give the structures of the following compounds. a....Ch. 6.2 - For each of the following compounds, A. give the...Ch. 6.3E - Prob. 6.4PCh. 6.4 - Prob. 6.5PCh. 6.5A - For each pair of compounds, predict which compound...Ch. 6.5B - Prob. 6.7PCh. 6.6B - Prob. 6.8PCh. 6.6B - The light-initiated reaction of...Ch. 6.6B - Show how free-radical halogenation might be used...
Ch. 6.7 - Prob. 6.11PCh. 6.7 - Prob. 6.12PCh. 6.8 - Prob. 6.13PCh. 6.9 - Predict the major products of the following...Ch. 6.9 - Prob. 6.15PCh. 6.10A - Prob. 6.16PCh. 6.11A - When diethyl ether (CH3CH2OCH2CH3) is treated with...Ch. 6.11B - Prob. 6.18PCh. 6.11B - For each pair of compounds, state which compound...Ch. 6.12 - Prob. 6.20PCh. 6.12 - Under appropriate conditions...Ch. 6.13 - Propose an SN1 mechanism for the solvolysis of...Ch. 6.13B - Prob. 6.23PCh. 6.13B - 3-Bromocyclohexene is a secondary halide, and...Ch. 6.15 - Prob. 6.25PCh. 6.15 - Prob. 6.26PCh. 6.16 - For each reaction, give the expected substitution...Ch. 6.16 - Prob. 6.28PCh. 6.16 - Prob. 6.29PCh. 6 - Prob. 6.30SPCh. 6 - Draw the structures of the following compounds. a....Ch. 6 - Give systematic (IUPAC) names for the following...Ch. 6 - Prob. 6.33SPCh. 6 - Predict the compound in each pair that will...Ch. 6 - Prob. 6.35SPCh. 6 - Give two syntheses for (CH3)2CHOCH2CH3, and...Ch. 6 - Prob. 6.37SPCh. 6 - Prob. 6.38SPCh. 6 - Chlorocyclohexane reacts with sodium cyanide...Ch. 6 - Give the substitution products expected from...Ch. 6 - Prob. 6.41SPCh. 6 - Prob. 6.42SPCh. 6 - Two of the carbocations in Problem6-42 are prone...Ch. 6 - Prob. 6.44SPCh. 6 - Predict the products of the following SN2...Ch. 6 - Prob. 6.46SPCh. 6 - Strawberry growers have used large quantities of...Ch. 6 - A solution of pure (S)-2-iodobutane ([]=+15.90) in...Ch. 6 - Prob. 6.49SPCh. 6 - Give a mechanism to explain the two products...Ch. 6 - Prob. 6.51SPCh. 6 - Because the SN1 reaction goes through a flat...Ch. 6 - Prob. 6.53SPCh. 6 - Furfuryl chloride can undergo substitution by both...Ch. 6 - Prob. 6.55SPCh. 6 - The following reaction takes place under...Ch. 6 - Propose mechanisms to account for the observed...Ch. 6 - Prob. 6.58SPCh. 6 - Prob. 6.59SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY