
Concept explainers
Ten mL of pure liquid water in a cylinder with a movable piston is heated at a constant pressure of 1 atm from an initial temperature of 80°C. The temperature of the system is monitored, and the following behavior is observed:
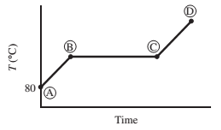
(a) What is happening in steps AB, BC, and CD? What is the temperature corresponding to the horizontal portion of the curve?
(b) Estimate the volume occupied by the water at points B and C. (Assume the vapor follows the ideal-gas equation of state.)
(a)
Interpretation:
Identify the process in AB, BC, CD areas and calculate horizontal zone temperature.
Concept introduction:
Energy is transferred into a system when it is heated. The system will change depending on the energy it receives. This can happen through increase in temperature. A heating curve is called a plot of the temperature versus time.
Answer to Problem 6.1P
- AB − Water liquid heating zone (temperature increase)
- BC − Vaporization of water (liquid to gas phase)
- CD − water vapor (gas phase) heating zone
Horizontal portion temperature = 100°C
Explanation of Solution
AB Step
As heat is absorbed, the temperature of the liquid begins to increase this is due to the increase in the kinetic energy of the molecules of liquid. For standard atmospheric pressure, the rise in temperature takes place until it reaches to 100°C. With increasing temperature, the volume of the liquid remains constant due to little expansion for liquids.
BC Step
At this point, the heat is consumed to begin vaporization of liquid. This temperature is known as the bubble point temperature. Due to change in the previous state, the temperature remains constant and water molecules are moving from liquid to vapor phase. At point C, the last drop of liquid gets evaporated.
CD Step
The temperature increases above 100 °C, when heating steam boils all the liquid into vapor. This cause increase in the volume. Step B shows transition of liquid to vapor. The boiling point of water is 100 °C (liquid here is water) and pressure is 1 atm.
(b)
Interpretation:
Volume occupied by water at point B and C should be calculated.
Concept introduction:
The ideal gas equation is represented as follows:
Here,
Answer to Problem 6.1P
At point B, the water is present as liquid only thus, the volume will be 10 mL
At point C
Explanation of Solution
At point B, the water is present as liquid only thus, the volume will be 10 mL
The number of moles in 10 mL of liquid water is calculated as follows:
The volume of vapor can be calculated using the ideal gas equation as follows:
Here, number of moles is 0.555 mol at 1 atm and
Putting the values,
Thus, at point C, the volume occupied by the water occupies is 17 L.
At point C,
Want to see more full solutions like this?
Chapter 6 Solutions
ELEM PRINC CHEM (LL) W/EBOOK
Additional Engineering Textbook Solutions
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Mechanics of Materials (10th Edition)
Starting Out with Python (4th Edition)
- Correctly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forward
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





