
Concept explainers
Ten mL of pure liquid water in a cylinder with a movable piston is heated at a constant pressure of 1 atm from an initial temperature of 80°C. The temperature of the system is monitored, and the following behavior is observed:
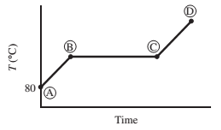
(a) What is happening in steps AB, BC, and CD? What is the temperature corresponding to the horizontal portion of the curve?
(b) Estimate the volume occupied by the water at points B and C. (Assume the vapor follows the ideal-gas equation of state.)
(a)
Interpretation:
Identify the process in AB, BC, CD areas and calculate horizontal zone temperature.
Concept introduction:
Energy is transferred into a system when it is heated. The system will change depending on the energy it receives. This can happen through increase in temperature. A heating curve is called a plot of the temperature versus time.
Answer to Problem 6.1P
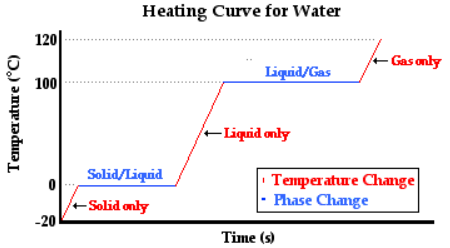
- AB − Water liquid heating zone (temperature increase)
- BC − Vaporization of water (liquid to gas phase)
- CD − water vapor (gas phase) heating zone
Horizontal portion temperature = 100°C
Explanation of Solution
AB Step
As heat is absorbed, the temperature of the liquid begins to increase this is due to the increase in the kinetic energy of the molecules of liquid. For standard atmospheric pressure, the rise in temperature takes place until it reaches to 100°C. With increasing temperature, the volume of the liquid remains constant due to little expansion for liquids.
BC Step
At this point, the heat is consumed to begin vaporization of liquid. This temperature is known as the bubble point temperature. Due to change in the previous state, the temperature remains constant and water molecules are moving from liquid to vapor phase. At point C, the last drop of liquid gets evaporated.
CD Step
The temperature increases above 100 °C, when heating steam boils all the liquid into vapor. This cause increase in the volume. Step B shows transition of liquid to vapor. The boiling point of water is 100 °C (liquid here is water) and pressure is 1 atm.
(b)
Interpretation:
Volume occupied by water at point B and C should be calculated.
Concept introduction:
The ideal gas equation is represented as follows:
Here,
Answer to Problem 6.1P
At point B, the water is present as liquid only thus, the volume will be 10 mL
At point C
Explanation of Solution
At point B, the water is present as liquid only thus, the volume will be 10 mL
The number of moles in 10 mL of liquid water is calculated as follows:
The volume of vapor can be calculated using the ideal gas equation as follows:
Here, number of moles is 0.555 mol at 1 atm and
Putting the values,
Thus, at point C, the volume occupied by the water occupies is 17 L.
At point C,
Want to see more full solutions like this?
Chapter 6 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Mechanics of Materials (10th Edition)
Starting Out with Python (4th Edition)
- 9.3. An ideal PD controller has the transfer function P Ke (TDs + 1) E An actual PD controller had the transfer function P = Ke E TDS +1 (TDIẞ)s +1 where ẞis a large constant in an industrial controller. If a unit-step change in error is introduced into a controller having the second transfer function, show that P(1) = Kc (1 + Ae˜¯BD) where A is a function of ẞwhich you are to determine. For ẞ=5 and K = 0.5, plot P(t) versus tl tp. As ẞ, show that the unit-step response approaches that for the ideal controller.arrow_forward9.1. A pneumatic PI temperature controller has an output pressure of 10 psig when the set point and process temperature coincide. The set point is suddenly increased by 10°F (i.e., a step change in error is introduced), and the following data are obtained: Time, s psig 0- 10 0+ 8 20 7 60 90 5 3.5 Determine the actual gain (psig per degree Fahrenheit) and the integral time.arrow_forward2. A unit-step change in error is introduced into a PID controller. If Ke TD = 0.5, plot the response of the controller P(t). = =10, 1, andarrow_forward
- A distribution of values is normal with a mean of 211 and a standard deviation of 50.4. Find the probability that a randomly selected value is between 59.8 and 155.6. P(59.8 X 155.6) = Enter your answer as a number accurate to 4 decimal places. Answers obtained using exact z-scores or z- scores rounded to 3 decimal places are accepted.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Depreciation and TaxesCalculate the depreciation using a suitable method (e.g., straight-line, declining balance) andincorporate tax implications based on current tax laws applicable to chemical plants. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Revenue EstimationEstimate the annual revenue based on the production capacity, product selling price, and marketdemand. Groups should also consider potential market fluctuations. Use following attached Process Flow Diagram as reference for this question.arrow_forward
- Topic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.TASKS:1) Capital Cost EstimationProvide a detailed breakdown of the initial capital investment, including land, building,equipment, and installation costs. Include any assumptions made in the estimation. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Breakeven Year CalculationUsing the cash flow analysis, calculate the breakeven year when the cumulative cash inflowequals the initial investment. Groups should graphically represent the breakeven point. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Cash Flow AnalysisDevelop a projected cash flow statement for the first 10 years of plant operation, consideringall the costs and revenues. Include working capital, loans, and interest payments if applicable. Use following attached Process Flow Diagram as reference for this question.arrow_forward
- Topic: Production of propylene glycol from glycerol derived from palm oil.QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Operational Cost AnalysisCalculate the yearly operational costs, including raw materials, labor, utilities, maintenance,and other recurring expenses. Provide a clear explanation of how these costs are derived. Use following attached Process Flow Diagram as reference for this question.arrow_forwardChemical Engineering Questionarrow_forwardA steam boiler or steam generator is a device used to produce steam by transferring heat to water. In our case, the combustion chamber is fueled with propane (C3H8) at a flowrate of 50.0 mol/h in an excess air of 50%. Assume that both propane and air are fed at 25ºC and the combustion gases leave the chamber at 200ºC. Pressure can be assumed to be atmospheric.* Determine: 1. The heat obtained assuming complete combustion. Compare the results using elements or compounds 2. The steam flowrate that could be generated if the heat is directed to obtain superheated steam at 2 bar and 160ºC from saturated liquid water at this pressure solvearrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





