
Chemistry: Structure and Properties, Books a la Carte PACKAGE W/MasteringChemistry, 2nd Edition
2nd Edition
ISBN: 9780134777559
Author: Tro, Nivaldo J.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 59E
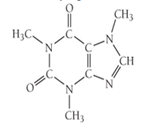
The structure of caffeine, present in coffee and many soft drinks, is shown here. How many pi bonds are present in caffeine? How many sigma bonds? Insert the lone pairs in the molecule. Which kinds of orbital do the lone pairs occupy?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
with full details solution please
write IUPAC names for these alcohols
Please list the String of Letters in the correct order.
Chapter 6 Solutions
Chemistry: Structure and Properties, Books a la Carte PACKAGE W/MasteringChemistry, 2nd Edition
Ch. 6 - Prob. 1ECh. 6 - What is a chemical bond according to valence bond...Ch. 6 - In valence bond theory, what determines the...Ch. 6 - In valence bond theory, the interaction energy...Ch. 6 - What is hybridization? Why is hybridization...Ch. 6 - How does hybridization of the atomic orbitals in...Ch. 6 - How is the number of hybrid orbitals related to...Ch. 6 - Sketch each hybrid orbital sp sp2 sp3 sp3d sp3d2Ch. 6 - Prob. 9ECh. 6 - Name the hybridization scheme that corresponds to...
Ch. 6 - What is a chemical bond according to molecular...Ch. 6 - Explain the difference between hybrid atomic...Ch. 6 - What is a bonding molecular orbital?Ch. 6 - Prob. 14ECh. 6 - What is the role of wave interference in...Ch. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - When applying molecular orbital theory to...Ch. 6 - In molecular orbital theory, what is a nonbonding...Ch. 6 - Write a short paragraph describing chemical...Ch. 6 - The valence electron configurations of several...Ch. 6 - The valence electron configurations of several...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Prob. 29ECh. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Which hybridization scheme allows the formation of...Ch. 6 - Which hybridization scheme allows the central atom...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Consider the structure of the amino acid alanine...Ch. 6 - Consider the structure of the amino acid aspartic...Ch. 6 - Sketch the bonding molecular orbital that results...Ch. 6 - Sketch the antibonding molecular orbital that...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Using the molecular orbital energy ordenng for...Ch. 6 - Using the molecular orbital energy ordering for...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - Draw an MO energy diagram for CO. (Use the energy...Ch. 6 - Draw an MO energy diagram for HCI. Predict the...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - Amino acids are biological compounds that link...Ch. 6 - The genetic code is based on four different bases...Ch. 6 - The structure of caffeine, present in coffee and...Ch. 6 - The structure of acetylsalicylic acid (aspirin) is...Ch. 6 - Draw a molecular orbital energy diagram for CIF....Ch. 6 - Draw Lewis structures and MO diagrams for CN+, CN,...Ch. 6 - Bromine can form compounds or ions with any number...Ch. 6 - The compound C3H4 has two double bonds. Describe...Ch. 6 - How many hybrid orbitals do we use to describe...Ch. 6 - Prob. 66ECh. 6 - In VSEPR theory, which uses the Lewis model to...Ch. 6 - The resuts of a molecular orbital calculation for...Ch. 6 - Prob. 69ECh. 6 - cis-2-Butene isomerizes (changes its structure) to...Ch. 6 - The ion CH5 + can form under very special...Ch. 6 - Neither the VSEPR model nor the hybridization...Ch. 6 - Prob. 73ECh. 6 - The most stable forms of the nonmetals in groups...Ch. 6 - Consider the bond energies of three iodine...Ch. 6 - How many atomic orbitals form a set of sp3hybrid...Ch. 6 - Have each group member pick one of these...Ch. 6 - Divide your group into two subgroups. Have one...Ch. 6 - A molecular orbital calculation for Hi results in...Ch. 6 - Determine the hybridization about 0 in CH3OH.Ch. 6 - Determine the hybridization about C in H2CO.Ch. 6 - According to the valance bond theory, which kind...Ch. 6 - Use molecular orbital theory to determine the bond...Ch. 6 - Use molecular orbital theory to predict which...Ch. 6 - Use molecular orbital theory to determine which...Ch. 6 - Which hybridization scheme occurs about nitrogen...Ch. 6 - Prob. 8SAQCh. 6 - Prob. 9SAQCh. 6 - Prob. 10SAQCh. 6 - Which type of orbitals overlap to form the sigma...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Propose an efficient synthesis for each of the following transformations. Pay careful attention to both the regio and stereochemical outcomes. ¡ H H racemicarrow_forwardZeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forward
- Saved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forwardDon't used Ai solutionarrow_forwardCalculate the differences between energy levels in J, Einstein's coefficients of estimated absorption and spontaneous emission and life time media for typical electronic transmissions (vnm = 1015 s-1) and vibrations (vnm = 1013 s-1) . Assume that the dipolar transition moments for these transactions are in the order of 1 D.Data: 1D = 3.33564x10-30 C m; epsilon0 = 8.85419x10-12 C2m-1J-1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY