
Concept explainers
Write the correct Lewis structure and assign a formal charge to each atom in fulminate ion, CNO−.
Interpretation:
The correct Lewis structure of fulminate ion
Concept Introduction:
Lewis structure otherwise known as Lewis dot diagrams or electron dot structures. The bond between atoms and lone pairs of electrons that is present in the molecule. Lewis structure represents each atom and their position in structure using the chemical symbol. Excess electrons forms the lone pair are given by pair of dots, and are located next to the atom.
The formula for the formal charge can be written as
Explanation of Solution
The correct Lewis structure of the fulminate ion
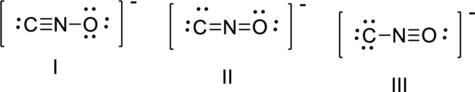
Achart can be drawn as,
The total number of valence electron is
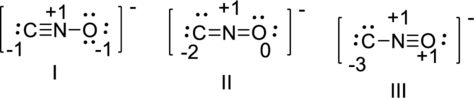
The Fulminate ion
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: The Molecular Science
- Using Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forwardThe molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forward
- BA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forward
- Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward1) NaNH2 (excess) 1) NaNH2 CI CI 2) H₂O 2) Mel 1) 03 2) (CH3)2S Na NH3 (liquid) 1arrow_forwardCI 1) n-BuLi 2) 1) 03 HH T&Cl 2) H₂O 2arrow_forward
- Help with a!arrow_forwardFor the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


