
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
4th Edition
ISBN: 9781260269284
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 42P
Interpretation Introduction
Interpretation:
Factors affecting the rate of a reaction needs to be determined.
Concept introduction:
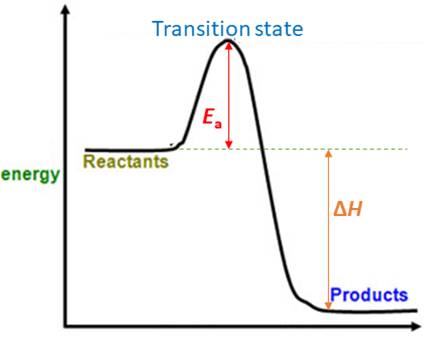
Enthalpy,
Energy difference between the reactants and the transition state is known as activation energy ( Ea , see the figure below). Activation energy, Ea is the minimum energy required by the reactants to initiate a reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
H
Draw the product and the mechanism
1.) LDA
+
2.) H30*
H
○ excess
OH
Given the first-order reaction: aA → products. State its kinetic equation.
Draw the product and the mechanism
excess
A.
ob
B.
C.
H*;
.OH
OH
H*:
✓ OH
H*; H₂O
D.
excess
8
1. LDA
2. H*
Chapter 6 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
Ch. 6.1 - Prob. 6.1PPCh. 6.1 - Prob. 6.1PCh. 6.1 - Prob. 6.2PPCh. 6.1 - Prob. 6.2PCh. 6.2 - Using the values in Table 6.2, give H for each...Ch. 6.2 - Prob. 6.4PPCh. 6.2 - Answer the following questions using the given...Ch. 6.2 - Given the H and balanced equation in Sample...Ch. 6.2 - Prob. 6.6PPCh. 6.3 - Prob. 6.7PP
Ch. 6.4 - Consider the reaction of ozone (O3) with nitrogen...Ch. 6.4 - Draw an energy diagram for an uncatalyzed...Ch. 6.5 - Identify the forward and reverse reactions in each...Ch. 6.5 - Write the expression for the equilibrium constant...Ch. 6.5 - Consider the reversible reaction AB, with K=1....Ch. 6.5 - Given each equilibrium constant, state whether the...Ch. 6.5 - Consider the following reaction:...Ch. 6.5 - Using the equilibrium mixture of reactants and...Ch. 6.5 - Calculate the equilibrium constant for each...Ch. 6.5 - Consider the representation depicted in the...Ch. 6.6 - Prob. 6.13PPCh. 6.6 - Prob. 6.14PPCh. 6.6 - wThe conversion of H2O to H2 and O2 is an...Ch. 6.6 - The reaction of O2 with NO to form NO2 and O2 is...Ch. 6.6 - wIn which direction is the equilibrium shifted in...Ch. 6.6 - Label each statement about the following...Ch. 6 - Prob. 11PCh. 6 - Prob. 12PCh. 6 - Prob. 13PCh. 6 - Prob. 14PCh. 6 - Prob. 15PCh. 6 - Prob. 16PCh. 6 - Prob. 17PCh. 6 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Prob. 21PCh. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Prob. 24PCh. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - Ammonia ( NH3 ) decomposes to hydrogen and...Ch. 6 - Prob. 29PCh. 6 - Ethanol ( C2H6O ), a gasoline additive, is formed...Ch. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Prob. 33PCh. 6 - Prob. 34PCh. 6 - Draw an energy diagram for the following reaction...Ch. 6 - Prob. 36PCh. 6 - State two reasons why increasing temperature...Ch. 6 - Why does decreasing concentration decrease the...Ch. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Which of the following affect the rate of a...Ch. 6 - Prob. 42PCh. 6 - How does a catalyst affect each of the following:...Ch. 6 - What is the difference between a catalyst and an...Ch. 6 - Prob. 45PCh. 6 - Consider the representation depicted in the...Ch. 6 - For each value, are the reactants or products...Ch. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Prob. 51PCh. 6 - Consider three different equilibrium mixtures...Ch. 6 - Write an expression for the equilibrium constant...Ch. 6 - Write an expression for the equilibrium constant...Ch. 6 - Prob. 55PCh. 6 - Use each expression for the equilibrium constant...Ch. 6 - Prob. 57PCh. 6 - Consider the following reaction:...Ch. 6 - Prob. 59PCh. 6 - Which of the following representations ([1][3]) of...Ch. 6 - Consider the following reaction....Ch. 6 - Consider the following reaction. H2(g)+I2(g)2HI(g)...Ch. 6 - Prob. 63PCh. 6 - Prob. 64PCh. 6 - Consider the reaction of N2(g)+O2(g)2NO(g). What...Ch. 6 - Consider the reaction of H2(g)+F2(g)2HF(g). What...Ch. 6 - Prob. 67PCh. 6 - Consider the reversible reaction ABA+B, shown at...Ch. 6 - Consider the endothermic conversion of oxygen to...Ch. 6 - Consider the exothermic reaction:...Ch. 6 - Consider the exothermic reaction:...Ch. 6 - Consider the endothermic reaction:...Ch. 6 - Consider the gas-phase reaction of ethylene...Ch. 6 - Methanol (CHO), which is used as a fuel in race...Ch. 6 - Prob. 75PCh. 6 - How does a catalytic converter clean up automobile...Ch. 6 - Prob. 77PCh. 6 - The reaction of salicylic acid with acetic acid...Ch. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83CPCh. 6 - Prob. 84CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forwardHow many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forward
- Which of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forward
- The structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forwardWhich of the following compounds would you most appropriately call hydrophobic? ○ CH4 H2CO CO HCI ○ NaClarrow_forwardWhich of the following triglycerides would you most expect to be a liquid at room temperature? saturated fat trans monounsaturated fat trans polyunsaturated fat cis monounsaturated fat ○ cis polyunsaturated fatarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY