
Concept explainers
(a)
Give estimation on weight and area of a typical car.
(a)
Answer to Problem 40PQ
A typical car can have weight around
Explanation of Solution
The average of weight of car can be taken as two thousand pounds. Express two thousand pounds in kilogram.
Normally a car can have
Conclusion:
Therefore, a typical car can have weight around
(b)
Draw the graph of
(b)
Answer to Problem 40PQ
Graph of
Explanation of Solution
The graph of
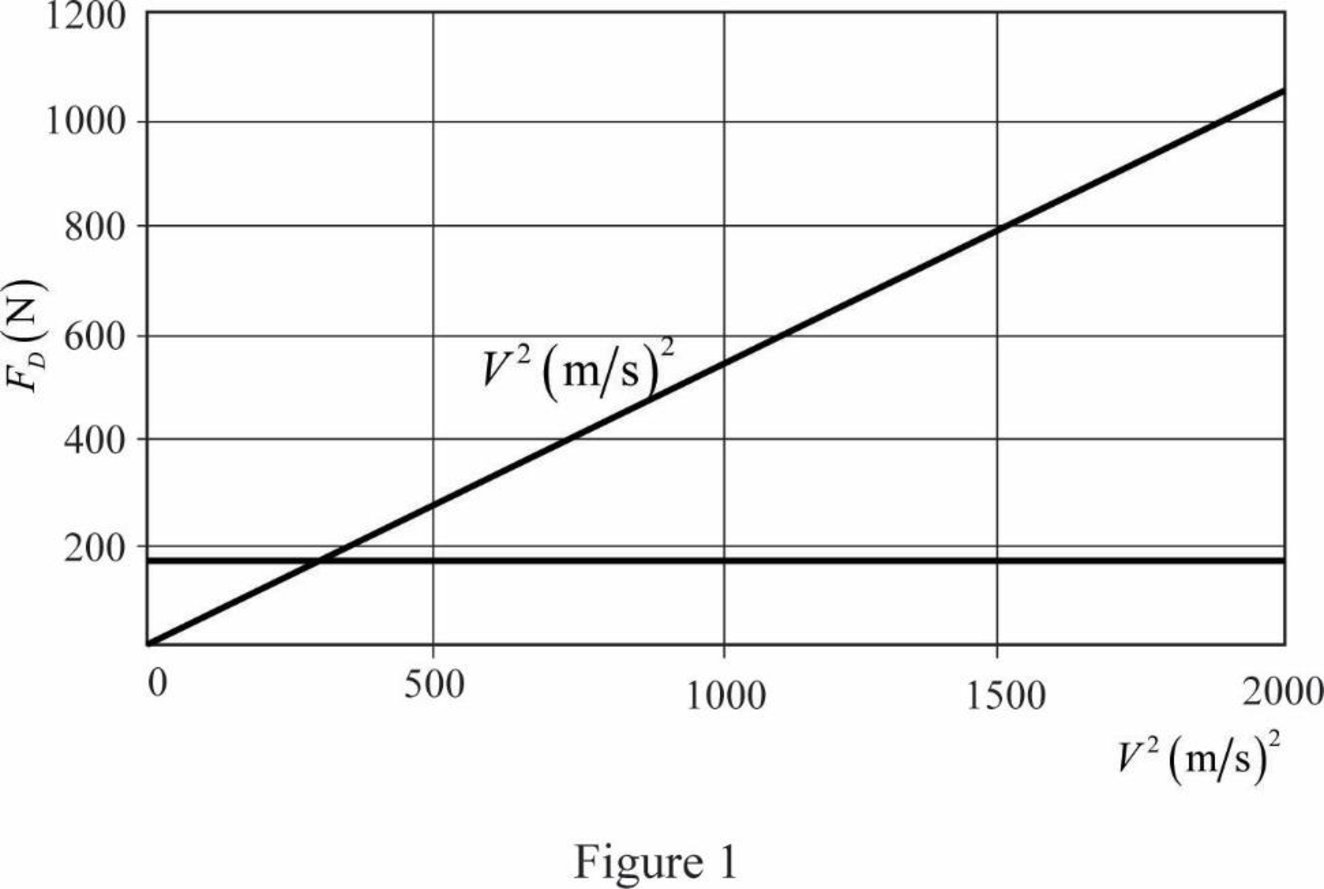
Write the equation for drag force.
Here,
Substitute
The equation tells that the drag force is varying with the square of speed of car. It can be seen that the graph is also conveying the same result. Graph shows that
Conclusion:
Therefore, the graph of
(c)
The value of Rolling
(c)
Answer to Problem 40PQ
Rolling frictional force is
Explanation of Solution
Write the equation to find the rolling friction.
Here,
Conclusion:
Substitute
It can be seen that
Therefore, the rolling frictional force is
(d)
The speed at which drag force equals rolling frictional force and the range of speed which the drag force can be neglected.
(d)
Answer to Problem 40PQ
The speed at which drag force equals rolling frictional force is
Explanation of Solution
Equate the right hand sides of equations (1) and (II).
Rewrite the above expression in terms of
It can be seen that lower speeds, drag force will be lesser than rolling frictional force. So drag force can be neglected at lower speed. It is better to limit the speed within
Conclusion:
Therefore, the speed at which drag force equals rolling frictional force is
(e)
The change in answer on the assumption that
(e)
Answer to Problem 40PQ
Explanation of Solution
Therefore, the
Want to see more full solutions like this?
Chapter 6 Solutions
Physics for Scientists and Engineers: Foundations and Connections
- (Figure 1)In each case let w be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w Find the direction of the force exerted on the strut by the pivot in the arrangement (a). Express your answer in degrees. Find the tension Tb in the cable in the arrangement (b). Express your answer in terms of w. Find the magnitude of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in terms of w.arrow_forward(Figure 1)In each case let ww be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w. Find the direction of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in degrees.arrow_forwardA 70.0 cm, uniform, 40.0 N shelf is supported horizontally by two vertical wires attached to the sloping ceiling (Figure 1). A very small 20.0 N tool is placed on the shelf midway between the points where the wires are attached to it. Find the tension in the left-hand wire. Express your answer with the appropriate units.arrow_forward
- Find the total bind Mev. binding energy for 13 Carbon, 6C (atomic mass = 13.0033554)arrow_forwardWhat is the 27 energy absorbed in this endothermic Auclear reaction 2] Al + 'n → 27 Mg + ! H? (The atom mass of "Al is 26.981539u. and that of 11 Mg is 26.984341u) MeVarrow_forwardWhat is the energy released in this nuclear reaction 1 F + "', H-1 O+ He? 19 19 16 (The atomic mass of 1F is 18.998403 u, and that of 20 is 15.9949154) MeV.arrow_forward
- What is the energy released in this B+ nuclear reaction خالد 2½ Al w/ Mg + ie? (The atomic mass of 11 Al is 23.9999394 and that > of 12 Mg is 23.985041 u) MeV.arrow_forwardWhat is the energy released / absorbed in this nuclear reaction 14 N+ & He → » O + ! N? (The atomic mass of 14 N is 14.003074u. 17N+ and that of 10 is 16.9991324). MeVarrow_forwardCan someone help me answer this question thanks.arrow_forward
- Can someone help me with this question thanks.arrow_forward4B. Four electrons are located on the corners of a square, one on each corner, with the sides of the square being 25 cm long. a) Draw a sketch of the scenario and use your sketch to b) Determine the total force (magnitude and direction) on one of the electrons from the other three?arrow_forwardPortfolio Problem 3. A ball is thrown vertically upwards with a speed vo from the floor of a room of height h. It hits the ceiling and then returns to the floor, from which it rebounds, managing just to hit the ceiling a second time. Assume that the coefficient of restitution between the ball and the floor, e, is equal to that between the ball and the ceiling. Compute e.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





