
(a)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of
(b)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
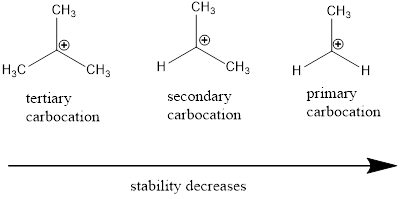
(c)
Interpretation:
The major product obtained from the acid catalyzed hydration of given alkene should be identified.
Concept introduction:
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
(d)
Interpretation:
The major product obtained from the acid catalyzed hydration of given alkene should be identified.
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
(e)
Interpretation:
The major product obtained from addition of
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
(f)
Interpretation:
The major product obtained from addition of
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
