
(a)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
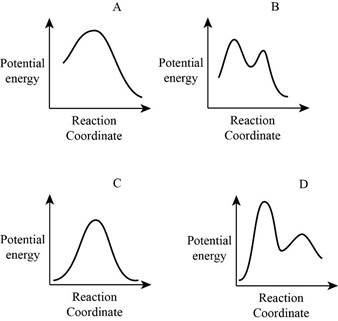
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(b)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
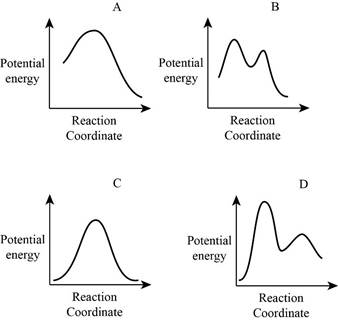
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(c)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
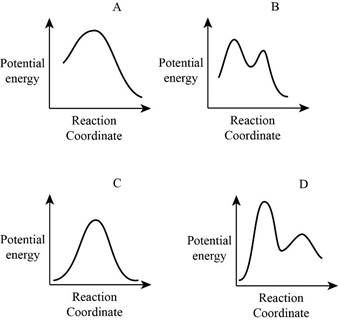
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(d)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
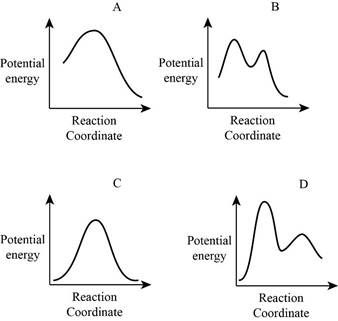
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(e)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(f)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(g)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(h)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY,SOLN.MAN.+...-ACCESS
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





