
Concept explainers
Assign the peaks in the
NMR spectrum of eugenol (Fig. 6.23) to specific protons in the molecule. The OH peak is at 5.1 ppm.
Interpretation:
The peak in
Concept introduction:
NMR is an analytical method that is utilized to identify the structure of a compound. It determines the framework of carbon-hydrogen bonds present in the compound.
Number of signals in the
The protons that are present in the different chemical environment are known as non-equivalent protons while the protons that are present in same chemical environment are known as equivalent protons.
Answer to Problem 1Q
Eugenol has 12 different hydrogen atoms. The three hydrogen atom present on the aromatic ring show peak around
Explanation of Solution
The structure of eugenol is as follows:
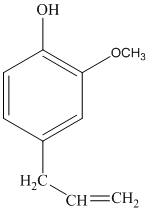
Eugenol has 12 different hydrogen atoms. The integration of peaks from right to left is 3:2:2:3:2.
There are two peaks for the three hydrogens around
There are two peaks for four hydrogen atoms around
There are two peaks for six around
Want to see more full solutions like this?
Chapter 6 Solutions
EBK MACROSCALE AND MICROSCALE ORGANIC E
- 2. Propose an efficient synthesis for each of the following transformations. Pay careful attention to both the regio and stereochemical outcomes. ¡ H H racemicarrow_forwardZeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forward
- Saved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forwardDon't used Ai solutionarrow_forwardCalculate the differences between energy levels in J, Einstein's coefficients of estimated absorption and spontaneous emission and life time media for typical electronic transmissions (vnm = 1015 s-1) and vibrations (vnm = 1013 s-1) . Assume that the dipolar transition moments for these transactions are in the order of 1 D.Data: 1D = 3.33564x10-30 C m; epsilon0 = 8.85419x10-12 C2m-1J-1arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





