
Review of Selected Concepts Related to Nomenclature
Write the chemical formula of each of the following. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
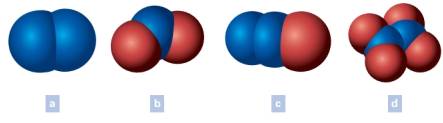
(a)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (a) is written as N2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. The number of each kind of atom which makes up the particle generally called as the composition, is denoted by the chemical formula. Commonly, elements can be madeup of molecules having single atom, two atoms or complex multi-atoms. Coversely, when the substance itself is an element it should have all atoms of the same element. In the above example (a), the two lobes are having the same blue color which is for the element nitrogen.
Total number of lobes of nitrogen (N) = 2
Chemical formula = N2
Thus, the chemical formula of the colored lobes (a) is written.
(b)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (b) is written as NO2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (b), the two lobes are having the same color which is for the element oxygen and one lobe is having the color for nitrogen.
Total number of lobes of nitrogen (N) = 1
Total number of lobes of oxygen (O) = 2
Chemical formula = NO2
Thus, the chemical formula of the colored lobes (b) is written.
(c)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (c) is written as N2O
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (c), the two lobes are having the same color which is for the element nitrogen and one lobe is having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 1
Chemical formula = N2O
Thus, the chemical formula of the colored lobes (c) is written.
(d)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (d) is written as N2O4
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (d), the two lobes are having the same color which is for the element nitrogen and other four lobes are having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 4
Chemical formula = N2O4
Thus, the chemical formula of the colored lobes (d) is written.
Want to see more full solutions like this?
Chapter 6 Solutions
Introductory Chemistry: An Active Learning Approach
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





