
In this chapter, we have learned about the
suppose it is hypothesized that it requires more energy to remove an electron from a metal that has atoms with one or more half-filled shells than from those that do not.
- Design a series of experiments involving the photoelectric effect that would test the hypothesis.
- What experimental apparatus would be needed to test the hypothesis? Its not necessary that you name actual equipment but rather that you imagine how the apparatus would work-think in terms of the types of measurements that would be needed, and what capability you would need in your apparatus.
- Describe the type of data you would collect and how you would analyze the data to see whether the hypothesis were correct.
- Could your experiments be extended to test the hypothesis for other parts of the periodic table, such as the lanthanide or actinide elements?
Interpretation: The experiments, apparatus and the type of data to test the given hypothesis is to be determined.
(a) A series of experiment involving the photoelectric effect that would test the hypothesis needs to be designed.
(b) The experimental apparatus required to test the given hypothesis should be determined.
(c)The type of data required to conclude whether the given hypothesis is correct or not should be determined.
(d) If the given hypothesis can be tested on lanthanides or actinides or not should be identified.
Concept Introduction: The light falls on the surface of the metal and results in the ejection of electron form its surface. This process is known as the photoelectric effect.
Answer to Problem 1DE
Solution: (a) When the light incident on the metal plate having one or more half-filled orbital; one does not observe the collection of electrons on the collector plate. But as the energy increases the electrons started collecting on the collector plate. This proves the given hypothesis.
(b) The experimental apparatus to study the photoelectric effect consists of the metal plate, collector plate, battery and voltmeter.
(c) The plot of stopping potential as a function of frequency is used to conclude that the given hypothesis is correct.
(d) The given hypothesis is not applicable on lanthanides and actinides because of the presence of d and f orbitals.
(a)
Explanation of Solution
The hypothesis says that that it requires more energy to remove an electron from the metal that has one or more half filled shells.
The ejection of electrons from the metal surface is tested by using the photoelectric effect by considering the apparatus consists of the metal plate on which the light is incident and the collector plate on which the electrons get collected. These two plates are connected to the electric circuit consists of battery, photodiode with amplifier and the voltmeter with reverse voltage.
When the light incident on the metal plate having one or more half filled orbital; one does not observed the collection of electrons on the collector plate. But as the energy increases the electrons starting collecting on the collector plate. This proves the given hypothesis.
(b)
Explanation:
The apparatus to study the photoelectric effect has the following parts,
- A photodiode with an amplifier.
- A digital voltmeter with reverse voltage.
- Batteries to operate amplifier and to provide reverse voltage.
- A monochromatic light source.
- The incident light beam intensity must adjust using a neutral filter.
The apparatus for testing the given hypothesis is shown below:
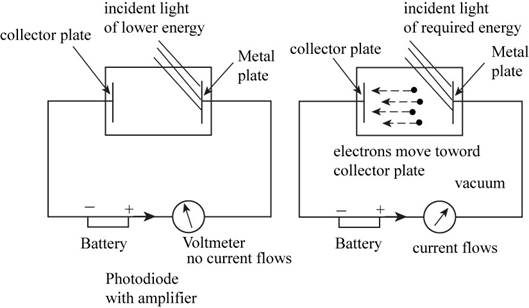
Figure 1
(c)
Explanation:
The data of frequency and wave length of different light source is collected and it is used in the apparatus of photoelectric effect. The different percentage transmission values as the function of intensity will be observed. The plot of stopping potential as a function of frequency will be observed from this data which conclude whether the hypothesis is correct or not.
(d)
Explanation:
The given hypothesis says that more energy is required to eject the electron form a metal having half filled orbitals as compared to those who have not. But the orbital of lanthanides and actinides are diffused in nature and they are larger in size. Therefore, they can easily accept and eject electrons by using lower energy radiation. Therefore, the given hypothesis cannot be tested on the lanthanides and actinides.
- The ejection of electrons is tested by using the apparatus consists of metal plate and collector plate.
- The experimental apparatus to study the photoelectric effect consists of the metal plate, collector plate, battery and voltmeter.
- The plot of stopping potential as a function of frequency is used to conclude that the given hypothesis is correct.
- The given hypothesis is not applicable on lanthanides and actinides because of the presence of d and f orbitals.
Want to see more full solutions like this?
Chapter 6 Solutions
CHM 101 VOL 1 2014 >IC<
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Campbell Biology (11th Edition)
Living By Chemistry: First Edition Textbook
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
- A compound has the molecular formula CH40, and shows a strong IR absorption at 2850-3150 cm. The following signals appear in the 'H NMR spectrum: 1.4 ppm (triplet, 6H), 4.0 ppm (quartet, 4H), 6.8 ppm (broad singlet, 4H). Which of the following structures is consistent with these data? Select the single best answer. OCH CH₂ x OCH2CH3 CH₂OCH3 OH CH₂OCH OH CH, OCH₁ CH₂OCH, CH₂OCH HO OH ° CH₂OCH3arrow_forwardpredict the major product while showing me the intermidiate products from each reagent/reagent grouparrow_forwardWhy is it desirable in the method of standard addition to add a small volume of concentrated standard rather than a large volume of dilute standard? An unknown sample of Cu2+ gave an absorbance of 0.262 in an atomic absorption analysis. Then 1.00 mL of solution containing 100.0 ppm (= µg/mL) Cu2+ was mixed with 95.0 mL of unknown, and the mixture was diluted to 100.0 mL in a volumetric flask. The absorbance of the new solution was 0.500. Calculate the concentration of copper ion in the sample.arrow_forward
- What is the relation between the standard deviation and the precision of a procedure? What is the relation between standard deviation and accuracy? The percentage of an additive in gasoline was measured six times with the following results: 0.13, 0.12, 0.16, 0.17, 0.20, 0.11%. Find the 90% and 99% confidence intervals for the percentage of the additive.arrow_forwardIf you measure a quantity four times and the standard deviation is 1.0% of the average, can you be 90% confident that the true value is within 1.2% of the measured average?arrow_forwardWrite down three most common errors in thermogravimetric analysis. Identify them as systematic or random errors and discuss how you can minimize the errors for better results.arrow_forward
- a) A favorable entropy change occurs when ΔS is positive. Does the order of the system increase or decrease when ΔS is positive? (b) A favorable enthalpy change occurs when ΔH is negative. Does the system absorb heat or give off heat when ΔH is negative? (c) Write the relation between ΔG, ΔH, and ΔS. Use the results of parts (a) and (b) to state whether ΔG must be positive or negative for a spontaneous change. For the reaction, ΔG is 59.0 kJ/mol at 298.15 K. Find the value of K for the reaction.arrow_forwardA sample of hydrated magnesium sulfate (MgSO4⋅xH2O) is analyzed using thermogravimetric analysis (TGA). The sample weighs 2.50 g initially and is heated in a controlled atmosphere. As the temperature increases, the water of hydration is released in two stages: (a) The first mass loss of 0.72 g occurs at 150°C, corresponding to the loss of a certain number of water molecules. (b) The second mass loss of 0.90 g occurs at 250°C, corresponding to the loss of the remaining water molecules. The residue is identified as anhydrous magnesium sulfate (MgSO4) Questions: (i) Determine the value of x (the total number of water molecules in MgSO4⋅xH2O) (ii) Calculate the percentage of water in the original sample. Write down the applications of TGA.arrow_forwardThe solubility product of iron(III) hydroxide (Fe(OH)3) is 6.3×10−38. If 50 mL of a 0.001 M FeCl3 solution is mixed with 50 mL of a 0.005 M NaOH solution, will Fe(OH)3 precipitate? Show all step-by-step calculations. To evaluate the equilibrium constant, we must express concentrations of solutes in mol/L, gases in bars, and omit solids, liquids, and solvents. Explain why.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





