
EBK CHEMISTRY FOR CHANGING TIMES
14th Edition
ISBN: 8220100663482
Author: MCCREARY
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 14P
Interpretation Introduction
Interpretation:
It should be predicted whether 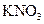 will dissolve in a non-polar solvent hexane or not.
will dissolve in a non-polar solvent hexane or not.
Concept introduction:
The very important concept of solubility is:
“like dissolves like”
This means that, a non-polar solute will dissolve in a non-polar solvent whereas a polar solute will dissolve in a polar solvent only.
To dissolve a substance in a solvent, the solute particles need to enter in the crystal lattice of the solvent. If, the solute is polar and solvent is non-polar, then the solute will not break into its species which could attach on solvent layers and hence the solute does not dissolve.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Draw the missing structure(s) in each of the following reactions. The missing
structure(s) can be a starting material or the major reaction product(s).
C5H10
H-CI
CH2Cl2
CI
Draw the products of the stronger acid protonating the other reactant.
དའི་སྐད”“
H3C
OH
H3C
CH
CH3
KEq
Product acid
Product base
Draw the products of the stronger acid protonating the other reactant.
H3C
NH2
NH2
KEq
H3C-CH₂
1.
Product acid
Product base
Chapter 6 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
Ch. 6 - Prob. 1RQCh. 6 - Prob. 2RQCh. 6 - Prob. 3RQCh. 6 - Prob. 4RQCh. 6 - Prob. 5RQCh. 6 - Prob. 6RQCh. 6 - Prob. 7RQCh. 6 - Prob. 8PCh. 6 - Prob. 9PCh. 6 - For which of the following would hydrogen bonding...
Ch. 6 - Prob. 11PCh. 6 - Prob. 12PCh. 6 - Prob. 13PCh. 6 - Prob. 14PCh. 6 - Prob. 15PCh. 6 - Prob. 16PCh. 6 - Prob. 17PCh. 6 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Prob. 21PCh. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Prob. 24PCh. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - Prob. 28PCh. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Prob. 33PCh. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - Prob. 36PCh. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Prob. 43PCh. 6 - Prob. 44PCh. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47APCh. 6 - Prob. 48APCh. 6 - Prob. 49APCh. 6 - Prob. 50APCh. 6 - Prob. 51APCh. 6 - Prob. 52APCh. 6 - Prob. 53APCh. 6 - Prob. 54APCh. 6 - Prob. 55APCh. 6 - Prob. 56APCh. 6 - Prob. 57APCh. 6 - Prob. 58APCh. 6 - Prob. 59APCh. 6 - Prob. 60APCh. 6 - Prob. 61APCh. 6 - Prob. 62APCh. 6 - Prob. 63APCh. 6 - Prob. 64APCh. 6 - Prob. 65APCh. 6 - Prob. 66APCh. 6 - Prob. 67APCh. 6 - Prob. 6.1CTECh. 6 - Prob. 6.2CTECh. 6 - Prob. 6.3CTECh. 6 - Prob. 6.4CTECh. 6 - Prepare a PowerPoint. poster, or other...Ch. 6 - Prob. 2CGPCh. 6 - Prob. 3CGPCh. 6 - Prob. 1CHQCh. 6 - Prob. 2CHQCh. 6 - Prob. 3CHQCh. 6 - Prob. 4CHQCh. 6 - Prob. 5CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forward
- Draw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward
- 3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Intermolecular Forces and Boiling Points; Author: Professor Dave Explains;https://www.youtube.com/watch?v=08kGgrqaZXA;License: Standard YouTube License, CC-BY