
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.3, Problem 5.6PP
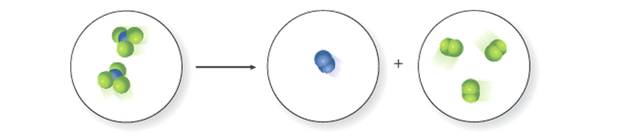
Write a balanced equation for the reaction depicted in the molecular art, and classify thereaction as a combination or decomposition.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.
Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Chapter 5 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 5.1 - Use the molecular art to identify the process as a...Ch. 5.1 - Use the molecular art to identify the process as a...Ch. 5.1 - Label the reactants and products, and indicate how...Ch. 5.1 - One term in a balanced chemical equation contained...Ch. 5.2 - Write a balanced equation for each reaction....Ch. 5.2 - Write a balanced equation for the following...Ch. 5.2 - Write a balanced equation for the reaction of...Ch. 5.2 - Consider the decomposition of hydrogen peroxide...Ch. 5.2 - Balance each chemical equation. Al+H2SO4Al2(...Ch. 5.3 - Write a balanced equation for the reaction...
Ch. 5.3 - Classify each reaction as a combination or...Ch. 5.3 - Classify each reaction as a single replacement or...Ch. 5.3 - Classify each reaction as a combination,...Ch. 5.3 - Fill in the needed reactants or products for each...Ch. 5.4 - Identify the species that is oxidized and the...Ch. 5.4 - Identify the species that is oxidized and the...Ch. 5.4 - (a) Write a balanced equation for the reaction...Ch. 5.4 - Prob. 5.10PCh. 5.5 - How many items are contained in one mole of (a)...Ch. 5.5 - How many carbon atoms are contained in each of the...Ch. 5.5 - How many molecules are contained in each of the...Ch. 5.5 - How many moles of water contain each of the...Ch. 5.6 - Calculate the formula weight of each ionic...Ch. 5.6 - The unmistakable odor of a freshly cut cucumber is...Ch. 5.6 - Prob. 5.11PPCh. 5.6 - Calculate the number of grams contained in each of...Ch. 5.6 - How many moles are contained in each of the...Ch. 5.6 - How many moles are contained in a 1,000.-mg dose...Ch. 5.6 - How many molecules are contained in two 500.-mg...Ch. 5.7 - Use the balanced equation for the reaction of N2...Ch. 5.7 - Use the balanced equation in Sample Problem 5.15...Ch. 5.8 - Prob. 5.16PPCh. 5.8 - Using the balanced equation for the combustion of...Ch. 5.8 - Prob. 5.17PPCh. 5.8 - Use the balanced equation, N2+O22NO, to answer the...Ch. 5.9 - What is the percent yield of X in a reaction that...Ch. 5.9 - Using the chemical equation in Sample Problem...Ch. 5.9 - Using the equation in Sample Problem 5.20, answer...Ch. 5.9 - The synthetic antiviral drug Tamiflu, currently...Ch. 5.10 - Consider the reaction of hydrogen and nitrogen to...Ch. 5.10 - Using the balanced equation for the reaction of H2...Ch. 5.10 - Using the balanced equation, 3H2(g)+N2(g)2NH3(g),...Ch. 5.10 - Using Sample Problem 5.21 as a guide, complete the...Ch. 5.10 - Using the balanced equation, N2(g)+O2(g)2NO(g),...Ch. 5.10 - Prob. 5.24PPCh. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - How many atoms of each element are drawn on each...Ch. 5 - How many atoms of each element are drawn on each...Ch. 5 - Use the molecular art to write a balanced equation...Ch. 5 - Use the molecular art to write a balanced equation...Ch. 5 - Use the molecular art to write a balanced equation...Ch. 5 - Some coal is high in sulfur (S) content, and when...Ch. 5 - Balance each equation. a....Ch. 5 - Balance each equation. a....Ch. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Prob. 36PCh. 5 - For the reaction depicted in the molecular art:...Ch. 5 - Answer the questions in Problem 5.37 for the...Ch. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Classify the reaction depicted in the molecular...Ch. 5 - Classify the reaction depicted in the molecular...Ch. 5 - Classify each reaction as combination,...Ch. 5 - Classify each reaction as combination,...Ch. 5 - Fill in the needed reactant or product for each of...Ch. 5 - Fill in the needed reactant or product for each of...Ch. 5 - Identify the species that is oxidized and the...Ch. 5 - Identify the species that is oxidized and the...Ch. 5 - Prob. 49PCh. 5 - Rechargeablenickel-cadmium batteries are used in...Ch. 5 - The reaction of hydrogen (H2) with acetylene...Ch. 5 - Prob. 52PCh. 5 - Calculate the formula weight and molar mass of...Ch. 5 - Calculate the formula weight and molar mass of...Ch. 5 - L-Dopa is a drug used to treat Parkinson’s...Ch. 5 - Niacin, vitamin B3, is found in soybeans, which...Ch. 5 - Which quantity has the greater mass? 1 mol of Fe...Ch. 5 - Prob. 58PCh. 5 - Mescaline is a hallucinogen in peyote, a cactus...Ch. 5 - Prob. 60PCh. 5 - How many grams are contained in 5.00 mol of each...Ch. 5 - How many grams are contained in 0.50 mol of each...Ch. 5 - A bottle of the pain reliever ibuprofen (C13H18O2,...Ch. 5 - One dose of Maalox contains 500. mg each of...Ch. 5 - How many moles are contained in each number of...Ch. 5 - How many moles are contained in each number of...Ch. 5 - How many molecules of butane (C4H10) are contained...Ch. 5 - Prob. 68PCh. 5 - The average nicotine (C10H14N2, molar mass 162.3...Ch. 5 - How many moles of sucrose...Ch. 5 - What is the mass in grams of each quantity of...Ch. 5 - What is the mass in grams of each quantity of...Ch. 5 - Using the balanced equation for the combustion of...Ch. 5 - Sodium metal (Na) reacts violently when added to...Ch. 5 - Prob. 75PCh. 5 - Using the balanced equation for the reaction of Na...Ch. 5 - What is the percent yield of B in a reaction that...Ch. 5 - What is the percent yield of B in a reaction that...Ch. 5 - The reaction of methane (CH4) with Cl2forms...Ch. 5 - Methanol (CH4O), which is used as a fuel in...Ch. 5 - Consider the given reaction mixture that contains...Ch. 5 - Consider the reaction of A2 and B2 to form A2B,...Ch. 5 - Prob. 83PCh. 5 - Consider the reaction with the balanced equation,...Ch. 5 - Using the balanced equation, 2NO+O22NO2, determine...Ch. 5 - Prob. 86PCh. 5 - Prob. 87PCh. 5 - Completer the followin table using the given...Ch. 5 - The local anesthetic ethyl chloride...Ch. 5 - The solvent dischloromethane...Ch. 5 - Answer the following questions about the...Ch. 5 - Answer the following questions about diethyl ether...Ch. 5 - Prob. 93PCh. 5 - Prob. 94PCh. 5 - Prob. 95PCh. 5 - Prob. 96PCh. 5 - TCDD, also called dioxin...Ch. 5 - Prob. 98CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
- Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY