
Concept explainers
Interpretation:
The suffix that is used to name the given compound has to be identified.
Concept Introduction:
All the molecules have their unique names. These names must be known in order to communicate. But remembering all the chemical names is impossible as there are many numbers of molecules. To avoid this, the
| Stereoisomerism | Substituents | Parent | Unsaturation | Functional Group |
Stereoisomerism indicate if the considered molecule has any stereocenters are present (R,S) and if double bond is present are cis/trans.
The groups that are connected to the main carbon chain is known as substituents.
The longest carbon chain is known as the parent.
Unsaturation indicates that if any triple or double bonds are present in the molecule.
Functional group is the one after which the considered compound is being named.
| Functional Group | Class of compound | Suffix |
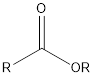 | Ester | -oate |
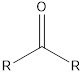 | -one | |
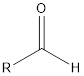 | -al | |
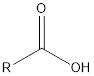 | -oic acid | |
 | Alcohol | -ol |
 | -amine |
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- Q1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward(4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forward
- Q2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forwardd) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forwardQ4: Predict the organic products for the following reactions. Then draw curved arrow electron- pushing mechanism for the reactions. a) NaBH4 EtOH Page 4 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) 요 1. Et₂O H MgBr 2. H+, H₂Oarrow_forward
- Would Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.arrow_forwardNH3 reacts with boron halides (BX3 where X = F, Cl, Br, or I) to form H3N-BX3 complexes.Which of these complexes will have the strongest N-B bond? Justify your answerarrow_forward3Helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





