
Concept explainers
Interpretation:
The partial molar volume of
Concept Introduction:
The partial molar volume is the contribution that a component of mixture makes to the total volume of a sample. The partial molar volume of a substance is expressed as,
Answer to Problem 5A.5P
The partial molar volume of
| 5 | 1.0151 | 0.006 | -15.946 |
| 10 | 1.107 | 0.012 | -10.357 |
| 15 | 1.167 | 0.0195 | -3.37075 |
| 20 | 1.23 | 0.027 | 3.6155 |
The graph between mole fraction of
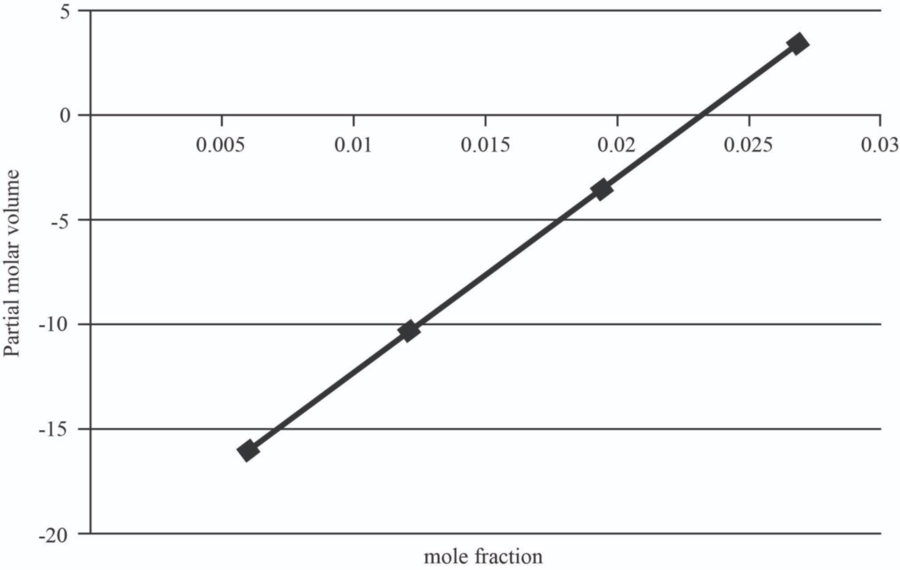
Figure 2
Explanation of Solution
The data of mass densities of aqueous solution of
| 5 | 1.0151 |
| 10 | 1.107 |
| 15 | 1.167 |
| 20 | 1.23 |
The moles of
The molar mass of
Substitute the molar mass of
The density of the solution (
Where,
Therefore, the molar density of the solution (
Where,
The volume of the solution is given by the formula below.
The mass of the solution is
Substitute the value of mass of solution in (3).
Substitute the value of
The mass of water (
Substitute the value of
The total molar volume of the solution (
Substitute the value of
The mass of
The density (
The molar mass of
The molar mass of
Substitute the value of
The mole fraction of
The mass of
Substitute the mass of
Similarly the values of
| 5 | 1.0151 | 0.006 | 17.94 |
| 10 | 1.107 | 0.012 | 17.86 |
| 15 | 1.167 | 0.0195 | 17.81 |
| 20 | 1.23 | 0.027 | 17.81 |
The values of
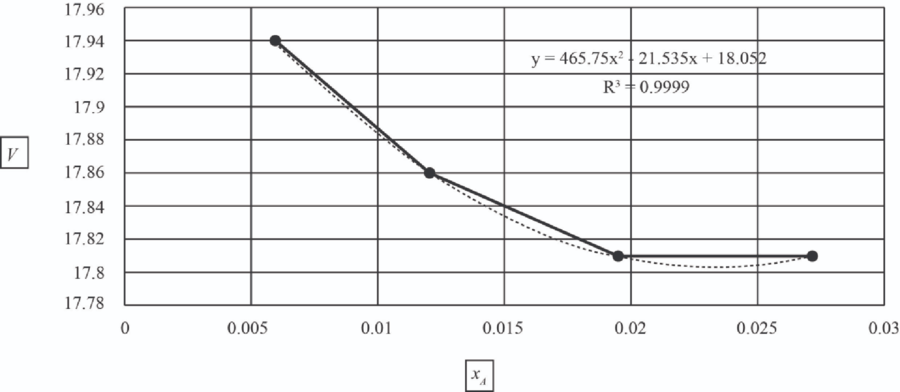
Figure 1
The data was fit to a second order polynomial using the POLY program to generate the equation given below.
The partial molar volume of a substance (
Where,
Differentiate equation (9) with respect to
Substitute the value of
Similarly the values of
| 0.006 | -15.946 |
| 0.012 | -10.357 |
| 0.0195 | -3.37075 |
| 0.027 | 3.6155 |
The graph between mole fraction of
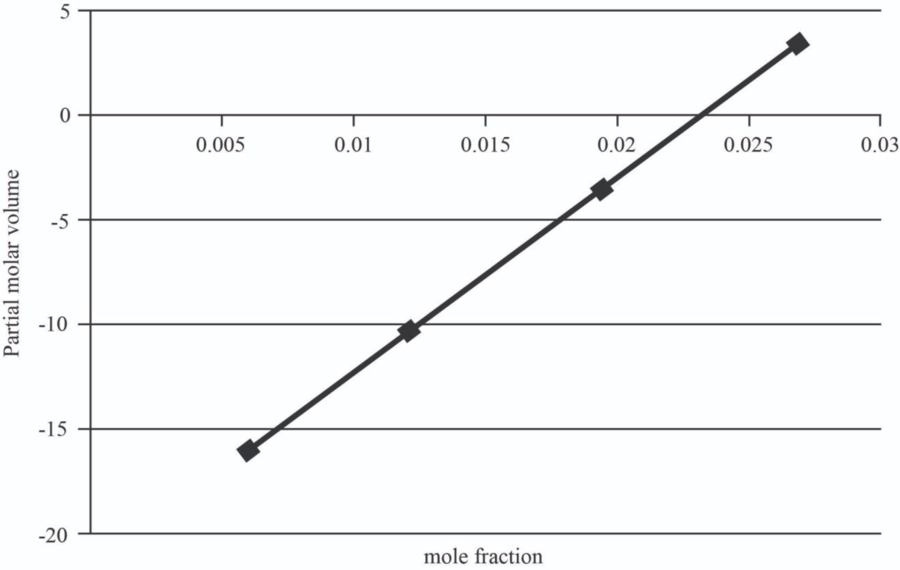
Figure 2
Want to see more full solutions like this?
Chapter 5 Solutions
Atkins' Physical Chemistry
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forward
- Draw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward
- 3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





