
5.53.20 Chemicals are stored in a laboratory with volume V(m3). As a consequence of poor laboratory practices, a hazardous species, A, enters the room air (from inside the room) at a constant rate 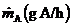 The room is ventilated with clean air ?owing at a constant rate
The room is ventilated with clean air ?owing at a constant rate 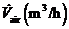 The average concentration of A in the room air builds up until it reaches a steady-state value
The average concentration of A in the room air builds up until it reaches a steady-state value 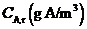
(a) List at least four situations that could lead to A getting into the room air.
(b) Assume that the A is perfectly mixed with the room air and derive the formula
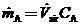
(c) The assumption of perfect mixing is never justi?ed when the enclosed space is a room (as opposed to, say, a stirred reactor). In practice, the concentration of A varies from one point in the room to another: it is relatively high near the point where A enters the room air and relatively low in regions far from that point, including the ventilator outlet duct. If we say that 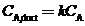 where
where 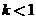 is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
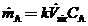
Use this equation and the ideal-gas equation of state to derive the following expression for the average mole fraction of A in the room air:
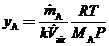
where MA is the molecular weight of A.
(b) The permissible exposure level (PEL) for styrene (M = 104.14) de?ned by the U.S. Occupational Safety and Health Administration is 50 ppm (molar basis).21 An open storage tank in a polymerization laboratory contains styrene. The evaporation rate from this tank is estimated to be 9.0 g/h. Room temperature is 20°C. Assuming that the laboratory air is reasonably well mixed (so that k = 0.5), calculate the minimum ventilation rate (m3/h) required to keep the average styrene concentration at or below the PEL. Then give several reasons why working in the laboratory might still be hazardous if the calculated minimum ventilation rate is used.
(e) Would the hazard level in the situation described in Part (d) increase or decrease if the temperature in the room were to increase? (Increase, decrease, no way to tell.) Explain your answer, citing at least two effects of temperature in your explanation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
ELEM.PRIN.OF CHEMICAL...ABRIDGED (LL)
Additional Engineering Textbook Solutions
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Database Concepts (8th Edition)
Degarmo's Materials And Processes In Manufacturing
Java: An Introduction to Problem Solving and Programming (8th Edition)
Electric Circuits. (11th Edition)
- =Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forward
- Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forwardBasic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning



